Organic chemistry study sheet
advertisement

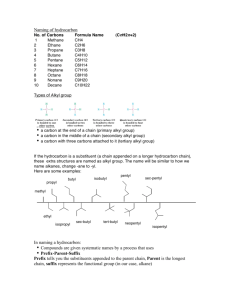
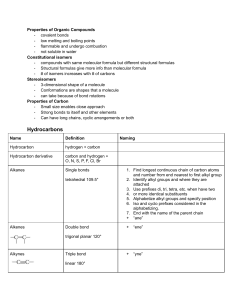
Organic Chemistry Study Sheet 1 carbon 2 3 4 5 6 7 8 9 10 Alkanes - “Saturated Hydrocarbons” All end in -ane. Contain all single bonds. Alkenes - “Unsaturated Hydrocarbons” All end in -ene. Contain at least one double bond, maybe more. Alkynes - “Unsaturated Hydrocarbons” All end in -yne. Contain at least one triple bond, maybe more. MethEthPropButPentHexHeptOctNonDec- General Naming Rules for the above 3: 1. Name the parent compound by finding the LONGEST chain of carbons (See chart for prefixes). 2. Name the groups attached to the chain by adding -yl to the end of our prefixes. (i.e. methyl, ethyl...) 3. Say how many groups there are (di-, tri-) 4. Tell which smallest number carbon the “alkyl” groups are on. Alkyl Halides A halogen atom replaces one of the Ketones hydrogens (F, Cl, Br, or I) O All end in -anone Prefixes go before the name of the parent chain: fluoro-, chloro-, bromo-, or Contain the carbonyl group: R C R’ iodo-. R and R’ are the parent carbon chain Alcohols (including the middle carbon under the All end in -anol. double-bond O) Contain the hydroxyl R OH Ethers group: The alkyl groups attached to the oxygen R group is the parent carbon chain. link are named in alphabetical order and Carboxylic Acids O followed by the word ether. All end in -anoic acid Contain the ether group: R–O–R Contain the carboxyl R C OH group: O Esters R group is the parent carbon chain Contain the ester group: R C O–R (including the carbon under the double All end in –anoate bond O) Name the alkyl group single bonded to Aldehydes O the oxygen, then the alkyl group that is All end in -anal. attached to the double-bonded carbon Contain the hydroxyl group: R H to the oxygen; the end is –anoate. R group is the parent carbon chain. General Naming Rules for the above 7 (except ethers/esters): 1. Name the parent compound by finding the LONGEST chain of carbons (See above chart for prefixes). 2. Number the carbons so you can determine how to number (with the lowest possible number) where the Alkyl halide/hydroxyl/carbonyl group is (Carboxyl and Aldehyde are ALWAYS on the end, so no number needed for that one only). Butanal Ethylmethyl ether Examples: Butanol Ethyl methanoate