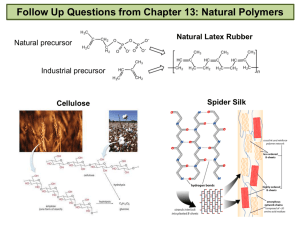
Organic Chemistry A Brief Introduction
advertisement
Organic Chemistry A Brief Introduction Text reference: Ch 20 • What isn’t it? • Organically grown fruits & veggies • It IS a branch of chemistry that deals with molecules containing the element carbon, bound to other elements. • Many (most) of these molecules are produced by living things (organisms organic) • So why do we call them “organically grown”? • What carbon-containing molecules can you think of (maybe from biology???) • Glucose C6H12O6 organic • Sucrose, dextrose and all the other “oses” • Sugars are organic • CO2 – carbon dioxide – inorganic • CO – carbon monoxide – inorganic • Most other carbon-containing molecules are organic • Many can be duplicated in laboratory • • • • • • • Any ideas? See p. 699 Fuel (from decayed living matter) Plastics PVC Velcro Artificial sweeteners • How many valence electrons does carbon have? • 4 • Carbon bonds readily with many, many, many other elements in a billion different ways. • It’s very versatile. • (BTW: Sulfur does the same thing in the nonliving world… but that’s the stuff of earth science) • The simplest of organic molecules. • What elements do you suppose hydrocarbons contain? • Hydrogen & carbon • Simplest of simplest = methane • Methane = one carbon atom, surrounded by 4 hydrogens, each sharing valence electrons • Organic molecules are bonded covalently • Lewis dot structures show shared electrons as dashes, instead of two dots. • What is the chemical formula for methane? • CH4 • Each carbon molecule has ____ bonding possibilities • 4 • In hydrocarbons, carbon bonds with itself and hydrogen • If the hydrocarbon is “saturated”, then all four available bonds of carbon are shared, or filled, by other atoms. • Saturated hydrocarbons are called alkanes • When naming, alkanes all end in “ane” • One carbon = methane • Two carbons = ethane • Ethane is a molecule made up of two carbons, surrounded by hydrogen using all the other available bonding spaces • What is the formula for ethane? • CH3CH3 # of carbons Name formula Structure diagram 1 Methane CH4 Draw these all in your 2 Ethane CH3CH3 notes, below your table 3 Propane CH3CH2CH3 Label them 4 Butane CH3CH2CH2CH3 5 Pentane CH3CH2CH2CH2CH3 6 Hexane CH3CH2CH2CH2CH2CH3 7 Heptane CH3CH2CH2CH2CH2CH2CH3 8 Octane CH3CH2CH2CH2CH2CH2CH2H3 9 Nonane CH3CH2CH2CH2CH2CH2CH2CH2CH3 10 decane CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 • • • • • (QUIZ TOMORROW ON PREFIXES and formulas) meth 1, starts with m, like mono does eth 2, follows meth, but isn’t mono, so drop the m prop- 3, How can you remember this? But- 4, rhymes with cute, which has four letters The rest of the prefixes are the same as the ones you’ve already learned for covalent naming • penta, 5, as in pentagon • hex – hex has an “x” like six does • hept - seven • oct – octopus has 8 legs • non- – sounds like nine • dec- – a decade has ten years • see board for this • To name these, add the prefix “cyclo” in front of the alkane name • ex: hexane becomes cyclohexane • When the attached groups are made up of hydrocarbons, these are called alkyl groups • Carbon groups attached to carbon chains are named by using the prefix for the number of carbons in the group + “yl” • Ex: methyl = CH3 attached to any carbon chain at any point. (see Table 20.2 on p. 709) • Find longest carbon chain • Number the carbons from the end closest to the first alkyl group • List alkyl groups in alpha order (disregarding prefix), with carbon number they’re attached to preceding group name • If more than one of the same kind of alkyl group exists, use di, tri, etc. prefixes in front of that alkyl group’s name • Ex: count the carbons in the longest chain: • • • • Eight carbons = octane Methyl group on carbon #4 Ethyl group also on carbon #4 4-ethyl, 4-methyl octane • HW: – Text p. 713 Practice Problems (answers in green section in back) – Text p. 714 Practice problem – Text p. 717 #8 a-d (watch out for bending carbon chains!)