Chapter 20
advertisement
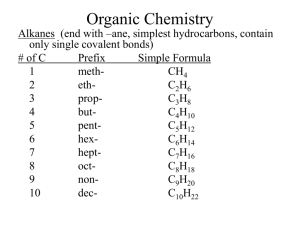
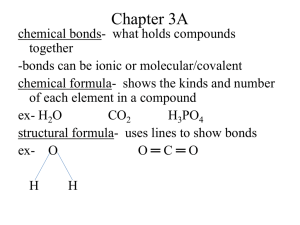
Chapter 25 Section 1 Organic Chemistry -study of compounds containing carbon hydrocarbons- organic compounds that only contain C and H *remember each carbon forms four bonds simple formula- shows the # and types of atoms structural formula- shows the #, type and arrangement of atoms molecular formula- shows the shapes of molecules ex- C4H10 -can also write condensed structural formula a different way CH3(CH2)2CH3 Alkanes (end with –ane, simplest hydrocarbons, contain only single covalent bonds) # of C Prefix Simple Formula 1 methCH4 2 ethC2H6 3 propC3H8 4 butC4H10 5 pentC5H12 6 hexC6H14 7 heptC7H16 8 octC8H18 9 nonC9H20 10 decC10H22 -all alkanes that are straight chains are named n-alkane (means normal) -look back at C4H10 example= n-butane Branched Chain Alkanes -alkane group takes the place of a hydrogen atom -called a substituent alkyl group- hydrocarbon substituent -ends with –yl (take away -ane) -carbon contains one less H -look at # of C to name ex- CH3 = methyl C2H5 = ethyl Naming Branched Chain Alkanes 1) Find the longest chain = parent molecule ex- heptane 2) # the C’s in the chain, making sure alkyl groups have lower numbers, can count backwards ex- 2,3,4 instead of 4,5,6 3) Add #’s to names of alkyl groups, with a dash in between ex- 2-methyl 3-methyl 4-ethyl 4) Use prefixes to denote multiples of the same alkyl groups, commas between #’s ex- 2,3-dimethyl 5) Put alkyl groups in alphabetical order ignoring prefixes ex- 4-ethyl-2,3-dimethyl 6) **Use proper punctuation -commas separate #’s -hyphens separate #’s and letters -no spaces 7) Add parent chain name ex- 4-ethyl-2,3-dimethylheptane *Remember prefixes* 2 = di3 = tri4 = tetra5 = penta6 = hexa7 = hepta8 = octa9 = nona10 = deca- Drawing Structural Formula From Name 1) Find parent chain and draw 2) # C’s on parent chain 3) Identify substituents and attach to proper C 4) Add hydrogen as needed Try These!! 1) 3-ethylhexane 2) 2,2,4-trimethylpentane 3) 3-ethyl-3,4-dimethyloctane