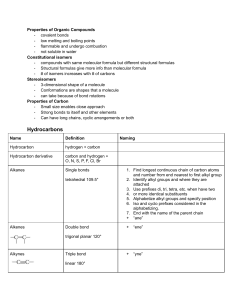
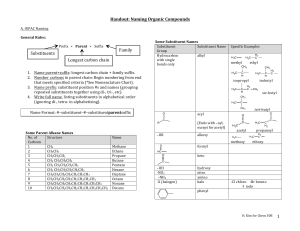
Naming of hydrocarbon In naming a hydrocarbon
advertisement

Naming of hydrocarbon No. of Carbons 1 Methane 2 Ethane 3 Propane 4 Butane 5 Pentane 6 Hexane 7 Heptane 8 Octane 9 Nonane 10 Decane Formula Name CH4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 (CnH2n+2) Types of Alkyl group • • • a carbon at the end of a chain (primary alkyl group) a carbon in the middle of a chain (secondary alkyl group) a carbon with three carbons attached to it (tertiary alkyl group) If the hydrocarbon is a substituent (a chain appended on a longer hydrocarbon chain), these extra structures are named as alkyl group. The name will be similar to how we name alkanes, change -ane to -yl. Here are some examples: propyl isobutyl butyl pentyl sec-pentyl methyl ethyl isopropyl sec-butyl tert-butyl neopentyl isopentyl In naming a hydrocarbon: • Compounds are given systematic names by a process that uses • Prefix-Parent-Suffix Prefix tells you the substituents appended to the parent chain, Parent is the longest chain, suffix represents the functional group (in our case, alkane) • Naming follows the IUPAC rules 1. Named as longest possible chain (one in red, 8 carbon, octane) 2. Carbons in that chain are numbered in sequence (this is consecutively) 3. substituents are numbered at their point of attachment (lowest possible number)start numbering on the end where the substituents will get the lowest number (3 methyl, not 6 methyl. 1 2 3 4 5 6 7 8 3-methyl octane 4. Di, tri, tetra, sec, tert are ignored in alphabetizing, iso, neo, cyclo are not ignored 1 2 3 4 5 6 7 8 3-ethyl-4,5-dimethyl octane 5. When both direction leads to the same lowest number for one of the substituents, the direction is chosen that gives the lowest possible number to one of the remaining substituents (2,2,4-trimethylpentane, not 2,4,4)