Ether PPT
advertisement

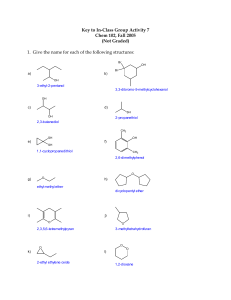
• General Formula: R1-O-R2 • Alkyl groups on both sides of Oxygen • Low chemical reactivity 1. Simple Ethers: Name alkyl groups A-Z followed by “ether”. Ex. CH3-O-CH2-CH3 “Ethyl methyl ether” 2. Another way is to add “-oxy-” to the prefix (meth-, eth-, etc) of the smaller carbon group. Ex. CH3-O-CH2-CH3 “methoxyethane” 3. If two alkyl groups are the same, use prefixes di-, tri-, tetra-, etc. to signify the branches. Ex. CH3-CH2-O-CH2-CH3 “Diethyl ether” or “ethoxyethane” • Also known as “Ether” or “Ethoxyethane” • Most common ether • Colorless and flammable liquid • Common solvent, general anesthetic Ethoxyethane • Shelf life of 2 years • Contact with air and light can form peroxide • Shake or Move= Boom Dimethyl Ether Ethylene Oxide Methoxybenzene • • • • • • Carboxylic Acids Esters Aldehydes Ketones Alcohols Amines • Ethers