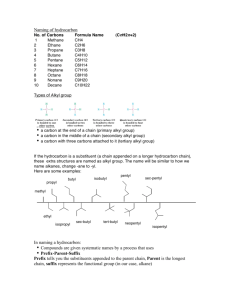
Elementary Organic Chemistry Chem 209 Exam I October 1, 2009
advertisement

Seat Assignment: _________________ Elementary Organic Chemistry Chem 209 Exam I October 1, 2009 Name (print): _____________________ Name (sign): _____________________ Student Number: __________________ Multiple Choice Answers (one letter per question, 3 points each) 5. _____________ 11. _____________ 6. _____________ 12. _____________ 7. _____________ 13. _____________ 8. _____________ 14. _____________ 9. _____________ 15. _____________ 10. _____________ 16. _____________ subtotal: _______________ Written Answer Questions (overpage, 4 points each): subtotal: __________________ Total: ________________ Please note: pencil is recommended! 1 Written Answer Questions (4 points each) 1. Draw, on the template, the structure of 2R,3R-dichloropentane. Use dashes and wedges to indicate absolute stereochemistry. 2. Draw the major product of the following reaction. 3. Draw the energy diagram for the reaction shown in question 2, above. Consider only the steps involving carbon atoms. 4. Draw in the box, by using a Newman projection, the highest energy (least stable) conformation of the C2–C3 bond of butane (CH3-CH2-CH2-CH3). Is it staggered or eclipsed? 2 Multiple Choice Questions (3 points each) 5. Give the major product. 6. Given the energy diagram below for the reaction of Br2/AlBr3 with benzene, identify the total energy change ∆E (released) for the reaction. A. B. C. D. E. a b c d e 3 7. For the reaction in question 6 above, how many steps are there, and how many intermediates? A. B. C. D. E. 8. What is the reason normally given for the observed order of stability of carbocations, 3° > 2° > 1° ? A. B. C. D. E. 9. 1 step, 1 intermediate 2 steps, no intermediates 2 steps, 1 intermediate 2 steps, 2 intermediates 3 steps, 2 intermediates alkyl groups are electron-releasing relative to H alkyl groups are electron-withdrawing relative to H alkyl groups destabilize a positive charge by resonance alkyl groups are more sterically hindered (larger) than H alkyl groups can exist in more conformations than H Which of the following is the most stable cyclohexadienyl cation? 4 10. Under acid catalysis, acetic acid reacts with alkenes. Draw the major product. Hint: this is a Markovnikov addition. 11. All of the carbons of which molecule, in its most stable conformation, lie in a single plane? A. B. C. D. E. cis-1,2-dichlorocyclopropane cyclobutane cyclopentane cyclohexane 1,1-dimethylcyclopropane 5 12. What is the formal charge on S? A. B. C. D. E. 13. +2 +1 0 -1 -2 What is the molecular formula for the line structure shown below? A. B. C. D. E. 14. C12H10 C12H15 C11H15 C11H10 C12H16 Arrange the following liquids in order of most-to-least soluble in hexane: I: n-decane A. B. C. D. E. 15. II: water III: n-butanol I > II > III I > III > II II > III > I III > I > II III > II > I Which of the following is achiral? A. B. C. D. E. (S)-lactic acid cis-1,2-dichlorocyclohexane 1-bromo-1-chloroethane 1-bromo-2-chloropropane cis-1-bromo-2-chlorocyclohexane 6 16. Which of the following has the smallest heat of hydrogenation per C=C bond (= total ∆Hhydrog / # of C=C’s)? Hint: this is a measure of C=C stability. A. B. C. D. E. 1-methylcyclohexene 1-methyl-1,3-cyclohexadiene 2-methyl-1,3-hexadiene 2-methyl-2-hexene toluene 7 8