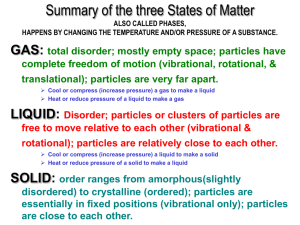
Unit 5 Objectives
advertisement

Unit 5 Objectives Students should be able to: 1. explain how a barometer works 2. calculate pressures for a closed and open end manometer 3. explain Boyle’s Law 4. explain Charles Law 5. explain Avogadro’s Law 6. apply the ideal gas equation to a variety of stoichiometric problems 7. apply the combined ideal gas equation to a variety of stoichiometric problems 8. apply the ideal gas equation to molar mass and density calculations 9. explain Dalton’s Law 10. apply Dalton’s Law to partial pressure and mole fraction problems 11. explain the process for collecting a gas over water and calculating its quantity 12. explain the assumptions for an “ideal” gas 13. apply kinetic molecular theory to explain physical observations 14. apply Graham’s Law of effusion to molecular mass calculations 15. explain deviations from ideal gas behavior 16. explain bond polarity 17. compare and contrast covalent network, ionic, covalent, dipole/dipole, hydrogen bonding and London dispersion forces 18 use kmt and concepts of intermolecular forces to make predictions of the macroscopic properties of gases in ideal and non-ideal situations 19 refine multiple representations of a sample of matter in the gas phase to accurately reflect the effect of changes in the macroscopic properties of the sample 20 describe a metallic bond 21 describe a phase diagram 22 describe gas chromatography and its relationship to intermolecular attractions. Explain how gas chromatography is used to separate samples of a gas mixture 23 explain how solutes can be separated by chromatography based on intermolecular interactions 24 design or interpret results of a separation experiment( filtration, paper chromatography, column chromatography, distillation) in terms of the relative strength of interactions among and between the components 25 explain the trends in properties and predict properties of samples consisting of particles with no permanent dipole on the basis of London dispersion forces 26 qualitatively analyze data regarding real gases to identify deviations from ideal behavior and relate these to molecular interactions 27 describe the relationships between the structural features of a polar molecules and the forces of attraction between the particles 28 explain the properties( phase, vapor pressure, viscosity, etc) of small and large molecular compounds in terms of the strengths and types of intermolecular forces 29 relate temperature to the motions of particles, either via particulate representations, such as drawings with arrows indicating velocities and/or via representations of average kinetic energy and distribution of kinetic energies of particles, such as plots of the Maxwell-Boltzman distribution 30 identify the noncovalent interactions within and between large molecules and/or connect the shape and function of the large molecule to the presence and magnitude of these interactions