Lab - Intermolecular Attractions
advertisement

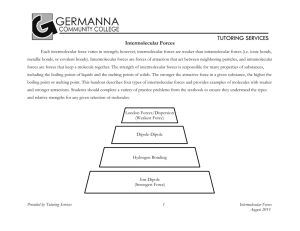
Name Period Date Pre-AP Chemistry Lab - Intermolecular Attractions In this experiment, temperature probes are placed in various liquids. Evaporation occurs when the probe is removed from the liquid’s container. This evaporation is an endothermic process that results in a temperature decrease. The magnitude of a temperature decrease is related to the strength of intermolecular forces of attraction. In this experiment, you will study temperature changes caused by the evaporation of several liquids and relate the temperature changes to the strength of intermolecular forces of attraction. The attractive force between molecules is based on polarity. A molecule which is polar has a “dipole” (+ and – parts). The bigger the dipole, the stronger the force between molecules. • Hydrogen bonds=A special case of a polar molecule. These are really strong in a molecule with an –OH part. An –OH at one end creates a more exposed (“sticky”) part than an –OH in the middle of the molecule. • Dipole Attraction=Any time there are two different types of atoms in a molecule, a small dipole is created. The bigger the difference in electronegativity, the larger the dipole. Again, dipoles near one end of a chain are exposed and are stickier than ones in the middle. • Induced Dipole = The weakest type of polar attraction. When any two molecules pass each other, the protons of one molecule pull on the electrons of the other. The more electrons there are, the larger this temporary dipole is. Basically, big molecules are stickier than small ones just because there are more electrons to get attracted to the other protons. Group 3 Group 2 Group 1 Data As you measure the temperature of an evaporating liquid, fill in the data table. Determine the molecular weight and structural formula of each molecule. A few examples have been done for you. Compound Formula methanol CH3OH ethanol C2H5OH 1-propanol C3H7OH 1-butanol C4H9OH acetone C3H6O 1-propanol C3H7OH 2-propanol C3H7OH pentane C5H12 1-butanol C4H9OH 2-butanol C4H9OH Water H2O Lab: Intermolecular Attractions Structural Formula Molecular Weight, u. Large, medium, small or zero dipole? Initial Temp; Tinitial, K Final Temp; Tfinal, K Change in Temp = Tfinal –Tinitial K 18.02 1 2/12/15 Analysis & Interpretation 1. Why does the temperature drop as the liquid evaporates? 2. ___________________ Does a large temperature drop correspond to strong or weak intermolecular forces? Explain. 3. (a) Based on your data and observations, rank the compounds from weakest to strongest intermolecular forces. Group 1 Group 2 Group 3 (b) What type(s) of intermolecular force play(s) the biggest role within this group? (c) What makes the molecule with the strongest force different from the others? 4. ___________________ Which compound (from this lab) has the strongest intermolecular forces? What observation supports this? Why does this compound have the strongest intermolecular forces? 5. ___________________ Which of these substances would have the highest vapor pressure? How can you tell from your data? 6. ___________________ Which of these substances would have the highest boiling point? Explain how you know. Lab: Intermolecular Attractions 2 2/12/15 Lab Instructions: Intermolecular Attractions PROCEDURE 1. Wear goggles! The compounds used in this experiment are flammable and poisonous. Avoid inhaling their vapors. Avoid contacting them with your skin or clothing. Be sure there are no open flames in the lab during this experiment. Notify your teacher immediately if an accident occurs. 2. A temperature probe should be plugged into in CH-1 3. Wrap the temperature probe with a square piece of filter paper secured by small rubber bands as shown. Roll the filter paper around the probe tip in the shape of a cylinder. The paper should be even with the probe end. 4. Obtain a vial with one of the samples. Clamp the vial to hold the it steady. 5. After the probe has been in the liquid for at least 45 seconds, press Start seconds to establish the initial temperature of each liquid. 6. Now remove the probe from the liquid and tape it so the probe tip extends 5 cm over the edge of the table top as shown. 7. After the data collection stops, find the change in temperature. Use the menu Analyze > Examine and hover over the temperature data to find the final temperature, T2, and the initial temperature, T1. Record these in your data table. 8. Subtract the initial temperature from the final temperature to determine ∆T, the temperature change during evaporation. (∆T=T2–T1) 9. Throw the filter paper into the garbage and prepare a new piece for the next trial. to begin data collection. Monitor the temperature for 15 10. Repeat this process with all the other samples. • If the compound appears twice in the table, use your previous data (don’t repeat the same liquid again). • Pressing the start button Lab: Intermolecular Attractions clears the old data. You don’t need a new file. 3 2/12/15 Group 3 Group 2 Group 1 Table with strutures Compound Formula methanol CH4OH ethanol C2H5OH 1-propanol C3H7OH 1-butanol C4H9OH acetone C3H6O 1-propanol C3H7OH 2-propanol C3H7OH pentane C5H12 1-butanol C4H9OH 2-butanol C4H9OH Water H2O Lab: Intermolecular Attractions Structural Formula Molecular Weight (mass of one mole) 4 Polar, mostly polar, somewhat polar or nonpolar Initial Temp; Tinitial Final Temp; Tfinal Change in Temp = Tfinal–Tinitial 2/12/15