Evaporation and Intermolecular Attractions
advertisement

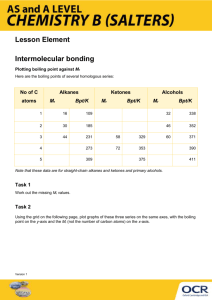
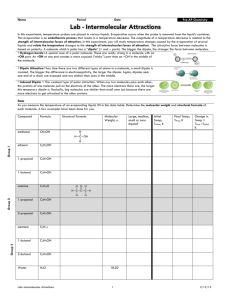
James Roberts Evaporation and Intermolecular Attractions Introduction In this demonstration, temperature probes are placed in various liquids. Evaporation occurs when the probe is removed from the liquid’s container. This evaporation is an endothermic process that results in a temperature decrease. The magnitude of a temperature decrease is, like viscosity and boiling temperature, related to the strength of intermolecular forces of attraction. In this demonstration two types of organic compounds will be encountered – alkanes and alcohols. In addition to carbon and hydrogen atoms, alcohols also contain the –OH functional group. This activity will demonstrate the molecular structure of alkanes and alcohols for the presence and relative strength of two intermolecular forces – hydrogen bonding and dispersion forces. Links to VCE Study Design Unit 1: Carbon Chemistry & Bonding Key Knowledge chemical language: symbols and formulas; empirical and molecular formulas; simple chemical equations; ethane as the first member of the homologous series of alkanes; names and molecular and empirical formulas of the alkanes and the corresponding alkenes up to C6; increase in melting temperature and boiling temperature with molecular size; structural isomers; structural formulas and systematic names of isomers C3H8 and C4H10; weak forces: permanent dipole–dipole interactions, including hydrogen bonding; dispersion forces; Key Skills collect numerical data generated by experiments; analyse/interpret experimental data; interpret and explain data relating to the physical properties of materials; predict properties of materials from a knowledge of the structure and bonding of material; Materials CBL system with Vernier Temperature Probes TI Graphing Calculator with appropriate cables and programs (with piggyback and overhead screen – optional) Small Filter Papers Small Elastic Bands methanol, ethanol, 1-propanol, 1-butanol, n-pentane, n-hexane (or parts thereof) Procedure Safety: Wear Goggles! The compounds used in this experiment are flammable and poisonous. Avoid inhaling vapours and avoid contact with skin or clothing. 1. Prepare the CBL unit, and TI graphing calculate. Attach probes and cables, make sure PYSICS program is loaded on the calculator. James Roberts 2. Run PYSICS/CHEMBIO program and SET UP PROBES and select USE STORED from the CALIBRATION menu. Set up the calculator to COLLECT DATA via a TIME GRAPH with suitable dimensions. It is recommended that you collect the data at intervals of 3 seconds over a period of 4 minutes (80 samples). (Running a live display is recommended for demonstrative purposes). 3. Wrap probe in filter paper secured by elastic bands. The paper should be even with the probe end. 4. Stand the probe in a test tube containing the liquid for 45 seconds and then press [enter] on the calculator to begin data collection. Don’t remove the probe yet. Monitor the temperature for 15 seconds to establish the initial temperature of each liquid. 5. Remove the probe and rest it securely so that the probe end is exposed to the air. 6. Find t (the temperature change during evaporation) by subtracting the minimum temperature from the maximum temperature. 7. Dispose of the filter paper appropriately and repeat the process for different liquids. 8. Complete the table and/or questions set by your teacher. Sample Questions 1. Two of the liquids, n-pentane and 1-butanol, had nearly the same molecular weights, but significantly different t values. Explain the difference in t values of these substances, based on their intermolecular forces. Even though n-pentane and 1-butanol have molecular weights of 72 and 74, respectively, 1butanol has a much smaller t due to the presence of hydrogen bonding between its molecules. This results in a stronger attraction and a slower rate of evaporation. 2. Which of the alcohols studied has the strongest intermolecular forces of attraction? The weakest intermolecular forces? Explain using the results of this experiment. The 1-butanol has the strongest attractions between its molecules. Methanol has the weakest attractions. The 1-butanol has the largest molecules and resulting strongest dispersion forces. This gives it the lowest evaporation rate and smallest t. 3. Which of the alkanes studied has the stronger intermolecular forces of attraction? The weaker intermolecular forces? Explain using the results of this experiment. The n-hexane has the stronger attractions between its molecules. The n-pentane has the weaker attractions. The n-hexane has the larger molecules and the resulting stronger dispersion forces. This gives it a lower evaporation rate and the smallest t. 4. Plot a graph of t values of the alcohols versus their respective molecular weights. Plot molecular weight on the horizontal axis and t on the vertical axis. Resources 1. Teacher Information – Evaporation and Intermolecular Attractions in Chemistry with CBL (comes with CBL kits) – Sample Questions/Table have been reproduced and the Procedure has been adapted for demonstrative purposes. 2. Chemistry VCE Study Design 3. Eltham High School – for their equipment and training.