Final Exam Study Guide Part 2- States of Matter Answer Key
advertisement

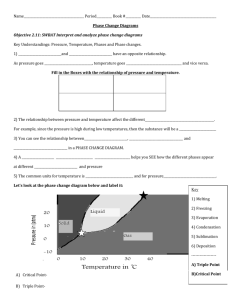
Change of Phase and Gas Law Review Sheet Define or describe the following in terms of energy: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Endothermic – absorbs energy (molecules speed up) Exothermic – releases energy (molecules slow down) Boiling – phase change from liquid to gas Condensation – phase change from gas to liquid Evaporation – phase change from liquid to gas Freezing – phase change from liquid to solid Heat of Fusion – Energy needed for melting /freezing Heat of Vaporization – Energy needed for boiling/condensing Melting – phase change from solid to liquid Sublimation- phase change from solid to gas Temperature- the measure of thermal energy of a substance Thermal energy- the internal kinetic energy of a substance Heat- the transfer of thermal energy from one object to another. Kinetic theory-explains how particles in matter behave. a) All matter is composed of small particles (atoms, molecules, and ions). b) These particles are in constant random motion. c) These particles collide with each other and the walls of their container. Phase Review Questions 1) What are the four phases of matter? Arrange them in order of most space between molecules/atoms to least space between molecules/atoms Plasma, Gas, Liquid, Solid Gas- no definite shape or volume; liquid- no definite shape, definite volume; solid- definite shape and volume 2) List the phases in order from slowest molecular movement to fastest molecular movement. Solid, liquid, gas 3) In which of the following phase changes are the molecules becoming less rigid in structure; condensation, evaporation, sublimation, boiling, melting, and freezing? Explain Moving to phase with less rigid bonds (more energy) 4) Does the temperature change during a phase change? Explain your answer. No: energy being used for change in state 5) Is energy absorbed or released in freezing? Released 6) Does sublimation increase or decrease the distance between particles? Give an explanation. Increase, Gas has fewer bonds (more energy) 7) If the temperature of a substance increases, what happens to its thermal energy? Increases 8) Describe freezing point, melting point, and boiling point. A) freezing point- the temperature at which a substance changes from liquid to solid B) melting point- the temperature at which a substance changes from solid to liquid C) boiling point- the temp at which a substance changes from liquid to gas Heating and Cooling Curve Review 12) In graph A, what phases are present at 50 °C? Liquid 13) How many phase changes take place in graph B? In graph C? What phase changes take place in graph D? B=1, C=2, D=0 (none in D) 14) In graph B, during what time interval is the substance entirely a solid? A liquid? 0-3, 8-9+ 15) What graph could represent pure water? Graph C (freezing and boiling pts the same) 16) Are any of the substances the same? How do you know? No – no phase change match 17) At what time in graph B has the substance just finished melting? 8 minutes 18. Calculate the amount of energy needed to increase the temperature of 22 grams of water from 50 degrees C to 115 degrees C. 19) Calculate the amount of energy needed to increase the temperature of 135 g water from -20 degrees to 120 degrees (as illustrated in graph C). 20) Label the following phase diagram with: solid, gas, liquid, critical point, triple point A: Solid B: Triple Pt C: Gas D: Liquid E: Critical Point Refer to the phase diagram below when answering the questions 19-24: 21) Draw a line at standard pressure and standard temperature. (0 degrees C and 1 atm) 22) What is the normal freezing point of this substance? 100 degrees C 23) What is the normal boiling point of this substance? 330 degrees C 24) If I had a quantity of this substance at a pressure of 1.25 atm and a temperature of 3000 C and lowered the pressure to 0.25 atm, what phase transition(s) would occur? Liquid to gas (vaporization or boiling) 24) At what temperature do the gas and liquid phases become indistinguishable from each other? 800 degrees C 25) If I had a quantity of this substance at a pressure of 0.75 atm and a temperature of -1000 C, what phase change(s) would occur if I increased the temperature to 6000 C? At what temperature(s) would they occur? Melting (90 degrees) and Boiling (or vaporization) (110 degrees) Gas Laws 1. Kelvin Scale K = C + 273.15 2. Describe Boyle’s Law: The volume of a given amount of gas held at constant temperature varies inversely with the applied pressure when temperature and mass are constant. Volume and Pressure are inversely proportional. When one goes up, the other goes down. Formula: P1V1 = P2V2 3. Describe Charles’ Law: The volume of gas held at constant pressure is directly proportional to the Kelvin temperature. Temperature and volume are directly proportional. If temperature increases, volume increases. Formula: V1/T1 = V2/T2 4. Describe Gay-Lussac’s Law Pressure of a given amount of gas held at constant volume is directly proportional to Kelvin temperature. Pressure and Temperature are directly proportional. If one goes up, so does the other. Formula: 5. Convert the following. 25 degrees C= ____________K 230K = ___________ degrees C 6) A sample of nitrogen gas has a volume of 150ml when it’s pressure is .947 atm. What will the volume of gas be at a pressure of .987 atm if temperature is constant? 7) A sample of helium gas is contained in a piston with a freely moving cylinder. At 0 degrees C, the volume of the gas is 375 mL. To what temperature must the gas be heated to occupy a volume of 500mL? 8) At 120 degrees C, the pressure of a sample of nitrogen is 1.07 atm. What will the pressure be at 205 degrees Celsius, assuming constant volume? 9. What is the ideal gas law? PV = nRT