PS.7
advertisement

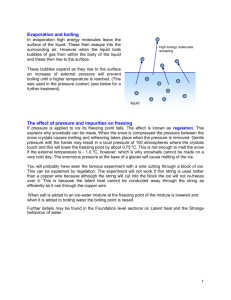
Name _______________________________ Date __________________ SOL PS.7 1. The temperature at which all molecular motion would stop is called: A B C D 0 degrees Celsius. absolute zero. 273 degrees Kelvin. absolute Kelvin. 2. The amount of heat required to change a solid to a liquid is called what? A B C D heat of vaporization heat of fusion heat of condensation specific heat 3. As a solid substance is heated, the vibration of its molecules increases. The molecules leave their fixed positions to move and slide over each other. What is happening to the substance? A B C D It is crystallizing. It is boiling. It is freezing. It is melting. 4. What will NOT happen when a gas is heated? A B C D The size of the gas molecules will increase. The kinetic energy of the gas molecules will increase. The temperature of the gas will increase. The pressure in a container holding the gas will increase. 5. What temperature is absolute zero? A B C D 0K – 273 K 0 oC 273 oC 6. What is the name of something that can reduce the heat flow between two materials? A B C D an isolator an insulator a resistance a reducer 7. In which direction does heat move? A B C D from lower temperature matter to higher temperature matter from higher temperature matter to lower temperature matter from matter at one temperature to other matter at similar temperatures in a direction to increase the temperature difference between the groups of matter 8. Why does the liquid in a thermometer work to indicate temperature? A B C D Liquids contract as their temperature increases so their volume decreases. The molecules condense as the temperature increases, increasing their volume. Liquids expand as their temperature increases, so their volume increases. The molecules evaporate as the temperature increases, decreasing their volume. _______________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott 15 Do not reproduce without permission. 07-15-00 Name _______________________________ Date __________________ SOL PS.7 9. In the metric system, the amount of heat necessary to change the temperature of 1 gram of water 1 oC is the: A B C D thermal unit. joule. calorie. Kelvin. 10. Heat transfer by radiation makes use of what types of waves? A B C D electromagnetic waves sound waves gamma waves pressure waves 11. In the winter, salt is often used to melt ice and snow. How does this process work? A The salt is heated by the sun. The heat transfers to the snow and ice and melts them. B Adding salt causes a heat-releasing chemical reaction to take place which melts the snow and ice. C Adding the salt lowers the freezing point of water below 0 °C and the snow and ice melt. 13. When you cook an egg in a frying pan, you are taking advantage of what type of heat transfer? A B C D radiation convection vaporization conduction 14. Boiling an egg makes use of which types of heat transfer? A B C D convection and radiation vaporization and conduction radiation and vaporization conduction and convection 15. If a pressurized can of spray paint is placed into a fire, what will probably happen? A B C D The can will contract and collapse. The can will explode. The can will melt. Nothing important will occur. D Adding the salt raises the boiling point of water above 0 °C, causing the snow and ice to melt. 16. Which of the following metals conducts heat the best? 12. What is the only type of heat transfer which can take place across a vacuum? A B C D A B C D lead iron aluminum copper radiation convection conduction No heat transfer can occur through a vacuum. _______________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott 16 Do not reproduce without permission. 07-15-00 Name _______________________________ Date __________________ SOL PS.7 Use the Phase Diagram below to answer the next two questions. Below is a diagram showing four parts of a heating sysytem. Phase Diagram 1 2 LIQUID SOLID D P r e s s u r e 3 4 C A B GAS 19. Which label(s) indicate the part(s) that make up the thermostat? Temperature 17. What are the proper labels for arrows A and B? A B C D A B C D 1 only 1 and 2 1 and 3 3 only A = boiling; B = freezing. A = evaporation; B = precipitation. A = sublimation; B = deposition. A = gasification; B = solidification. 18. What are the proper labels for arrows C and D? A B C D C = precipitation; D = solidification. C = condensation; D = freezing. C = evaporation; D = sublimation. C = boiling; D = desposition. _______________________________________________________________________________________________________ Copyright©1999,2000 S.S. Flanagan & D.E. Mott 17 Do not reproduce without permission. 07-15-00