Name_______________________________________
advertisement
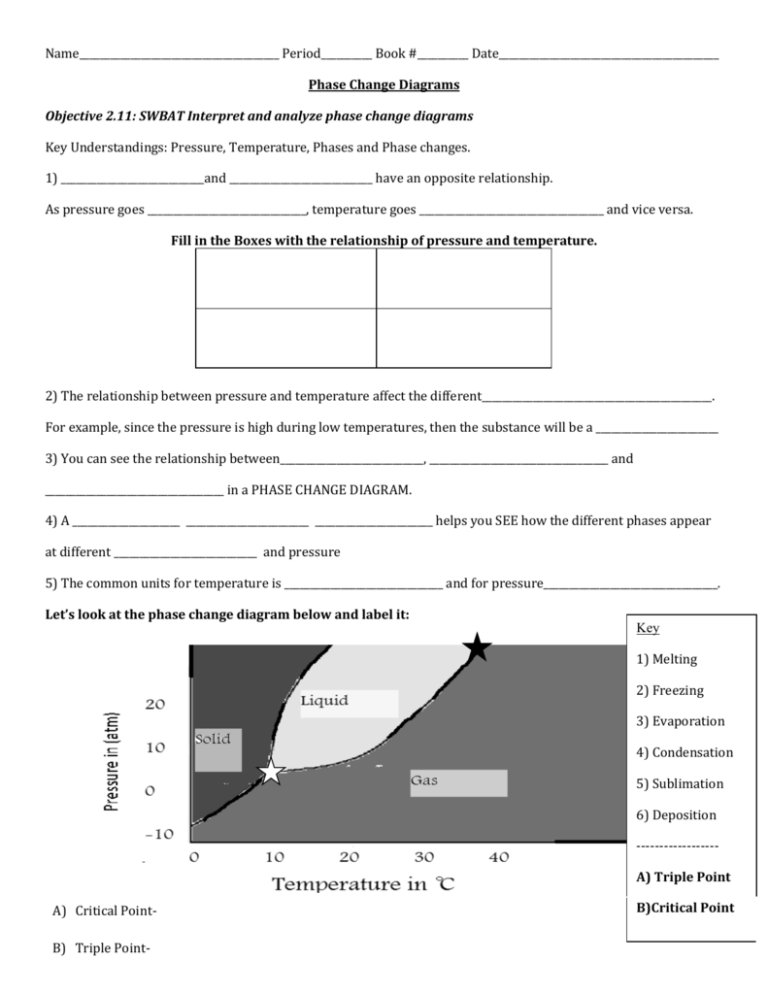
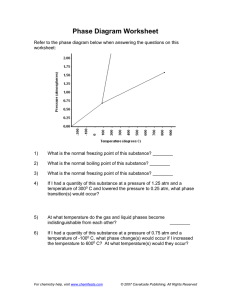
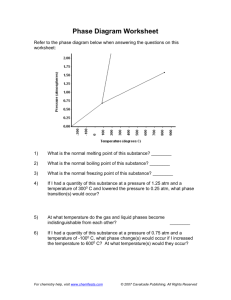
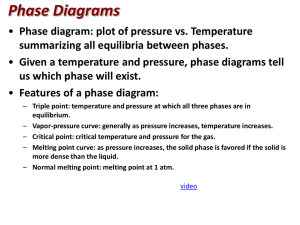
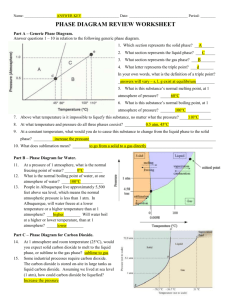
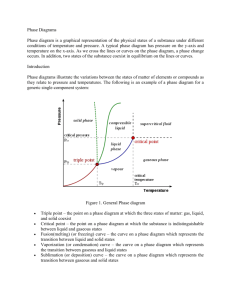
Name_______________________________________ Period__________ Book #__________ Date___________________________________________ Phase Change Diagrams Objective 2.11: SWBAT Interpret and analyze phase change diagrams Key Understandings: Pressure, Temperature, Phases and Phase changes. 1) ____________________________and ____________________________ have an opposite relationship. As pressure goes _______________________________, temperature goes ____________________________________ and vice versa. Fill in the Boxes with the relationship of pressure and temperature. 2) The relationship between pressure and temperature affect the different_____________________________________________. For example, since the pressure is high during low temperatures, then the substance will be a ________________________ 3) You can see the relationship between____________________________, ___________________________________ and ___________________________________ in a PHASE CHANGE DIAGRAM. 4) A _____________________ ________________________ _______________________ helps you SEE how the different phases appear at different ____________________________ and pressure 5) The common units for temperature is _______________________________ and for pressure__________________________________. Let’s look at the phase change diagram below and label it: Key 1) Melting 2) Freezing 3) Evaporation 4) Condensation 5) Sublimation 6) Deposition -----------------A) Triple Point A) Critical PointB) Triple Point- B)Critical Point 6) How can you tell the freezing point and boiling point of a substance with a phase change diagram? Look at the phase change diagram of water below. Liquid water 7) When the Pressure is at __________________________ you can follow the line and find what _____________________________ the substance freezes and boils at. Key Understandings- Checking for Understanding 8) What temperature does water freeze at? 9)What temperature does water boil at? 10) At what temperature and pressure does water exist as a solid, liquid and a gas? 11) At what temperature and pressure is water’s critical point? 12) What is a critical point? Phase Diagram Worksheet Look at the phase diagram below to answer the questions on this worksheet about the mystery substance. Liquid Solid Gas 1) What is the normal freezing point of this substance? ________ 2) What is the normal boiling point of this substance? ________ 3) If I had a quantity of this substance at a pressure of 1.25 atm and a temperature of 3000 C and lowered the pressure to 0.25 atm, what phase change would happen? 5) At what temperature is the critical point? 6) If I had a quantity of this substance at a pressure of 0.75 atm and a temperature of -1000 C, what phase change(s) would occur if I increased the temperature to 6000 C? Phase Diagram Worksheet Answers Refer to the phase diagram below when answering the questions on this worksheet: 1) What is the normal freezing point of this substance? 1000 C 2) What is the normal boiling point of this substance? 3750 C 3) What is the normal freezing point of this substance? 1000 C 4) If I had a quantity of this substance at a pressure of 1.25 atm and a temperature of 3000 C and lowered the pressure to 0.25 atm, what phase transition(s) would occur? It would vaporize at a pressure of ~0.75 atm. 5) At what temperature do the gas and liquid phases become indistinguishable from each other? 8250 C 6) If I had a quantity of this substance at a pressure of 0.75 atm and a temperature of -1000 C, what phase change(s) would occur if I increased the temperature to 6000 C? At what temperature(s) would they occur? Name_______________________________________ Period__________ Book #__________ Date__________________________________ Phase Change Diagrams Objective 2.11: SWBAT Interpret and analyze phase change diagrams Key Understandings: Pressure, Temperature, Phases and Phase changes. 1) ____Pressur e____and ____temperature__________ have an opposite relationship. As pressure goes ________up___________, temperature goes _________down___________ and vice versa. Fill in the Boxes with the relationship of pressure and temperature. 2) The relationship between pressure and temperature affect the different ____states of matter______________. For example, since the pressure is high during low temperatures, then the substance will be a _____solid__ 3) You can see the relationship between______solid___, ____________liquid_____ and _____gas___________________ in a PHASE CHANGE DIAGRAM. 4) A _____phase______ ______change______ ___diagram________ helps you SEE how the different phases appear at different _____temperatures________ and pressure 5) The common units for temperature is ______Celsius (C°)________ and for pressure_____atm______________. Let’s look at the phase change diagram below and label it: Key 1) Melting 2) Freezing 3) Evaporation 4) Condensation 5) Sublimation 6) Deposition -----------------A) Triple Point C) Critical Point-Point at which a substance is a gas no matter the pressure D) Triple Point- Point at which a substance is a solid, liquid and a gas at the same time B)Critical Point