PROPERTIES OF MATTER
advertisement

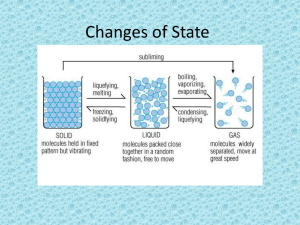
PROPERTIES OF MATTER MATTER • What is matter? • States (phases) of matter • Properties of matter? – Physical properties – Chemical properties – Characteristic properties • Changes in matter • Types of matter What is Matter? Matter is the “stuff” that makes up everything. Matter is anything that has mass and takes up space (volume). Therefore matter is anything that has DENSITY! States of Matter SOLIDS What do you think the particles inside a solid are arranged like? How do you think the particles inside the solid move like? States of Matter Liquids What do you think the particles inside a liquid are arranged like? How do you think the particles inside the liquid move like? States of Matter GASES What do you think the particles inside a gases are arranged like? How do you think the particles inside the gases move like? States of Matter SOLID Melting Point Freezing Point LIQUID Condensation Point Boiling Point GAS WHAT IS SUBLIMATION? PROPERTIES OF MATTER Physical Properties Can be observed or measured without changing the chemical makeup of the substance. Examples include: Color, Density, Taste, Texture, Mass Chemical Properties Can be observed only in chemical reactions involving the substance. Examples include: Ability to rust, flammability Characteristic Properties Are very specific to each type of matter, like fingerprints. They are UNIQUE to that substance and are used to identify matter. Examples include: density,melting, boiling and freezing points, and conductivity PROPERTIES OF MATTER LETS REVIEW! QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Qui ckTi me™ and a TIFF (Uncompress ed) decompres sor are needed to s ee this pi cture. Melting point, boiling point, and freezing point are all examples of what kind of property? Why? CHARACTERISITC PROPERTIES BECAUSE THEY ARE UNIQUE TO THAT SUBSTANCE PROPERTIES OF MATTER LETS REVIEW! Shape, volume, and mass are examples of what kind of property and why? PHYSICAL PROPERTY because it is used to describe a substance BUT it is not a characteristic property because these things are not unique to that substance. PROPERTIES OF MATTER LETS REVIEW! Flammability, rusting, reacting with water are examples of what kind of property? CHEMICAL PROPERTY because it is used to describe a substances ability to react with another substance. PHYSICAL PROPERTIES • Besides density, boiling point freezing point, melting point, condensation, taste, color…. Are there any other examples of physical properties? Malleability Hardness Ductility Elasticity Conductivity Solubility CHANGES IN MATTER PHYSICAL AND CHEMICAL CHANGES A physical change is when there is a change in the matter but NO NEW substances are formed. What are some examples? Usually there is a change in the SIZE, SHAPE, or STATE that the matter is in… remember… no new substances are formed/produced! HINT: Usually, physical changes are reversible CHANGES IN MATTER • A chemical change is a change in matter where NEW substances are formed. • How do you know that a new substance has formed? The properties of the matter have changes! Like …color, density, boiling pt, solubility, etc. HINT! Usually, chemical changes are NOT reversible. CHANGES IN MATTER LET’S REVIEW! It is sometimes difficult to tell whether a chemical or a physical change has occurred. Here are some clues that might help: » Burning always indicates chemical change » If a NEW color appears, it is a chemical change » A change in the state of the matter indicates a physical change. Do not get confused with gas production which is a chemical change. » Dissolving is a physical change…it is reversible