Phase Changes
advertisement
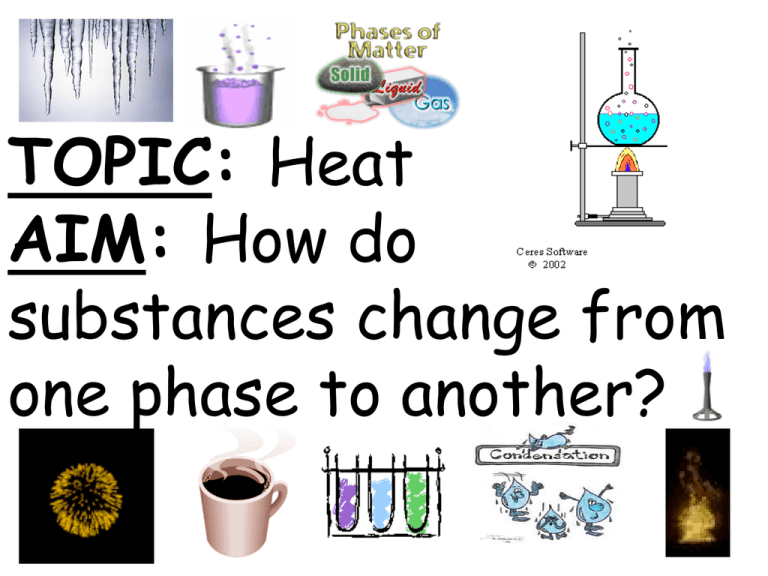
TOPIC: Heat AIM: How do substances change from one phase to another? States • (phases) • of matter • 1. SOLID Molecules packed closely together Vibrate in place • Definite volume & shape • 2. LIQUID • Molecules farther apart & move around • Definite volume • No definite shape • 3. GAS • Molecules very far apart & move around very fast • A lot of empty space (smaller density) • No definite shape or volume • 4. PLASMA • High energy molecules • Sun, stars, lightening, fluorescent & neon lights Can you find the different phases of matter in this picture? 2 types of changes • 1. Physical change • Phase change • Does not produce a new substance • (change in appearance) • Examples: freezing, melting, evaporation, tearing, crushing • 2. Chemical Change • Produces a NEW substance • Examples: burning, rusting Paper ash, smoke, heat Iron + oxygen iron oxide (rust) Raw egg cooked egg Cake batter Cake Sodium + Chlorine NaCl = Sodium Chloride (salt) Phase change • Physical change • Requires heat energy Phase Changes Freezing • Liquid Solid • Heat removed Melting • Solid Liquid • Heat added Evaporation • Liquid Gas • Heat added • Vaporization Condensation • Gas Liquid • Heat removed •Opposite of evaporation Sublimation • Solid Gas • Heat added • Example: Dry ice (solid CO2 gas) Solid Gas Gas Crystals Example: Iodine crystals gas (slightly above room temp) PHASE CHANGES Add heat Energy absorbed by molecules Melting SL SOLID Evaporation LG LIQUID Freezing LS GAS Condensation GL Remove heat Energy released by molecules Freezing • Temp at which a liquid freezes (LS) point • FP of water = 0°C Melting • Temp at which a substance melts (SL) point • MP of water = 0°C • Freezing Pt = Melting Pt Boiling • Temp at which a point substance evaporates (LG) • BP of water = 100°C Evaporation occurs at the surface of a liquid (left). When a liquid boils, gas bubbles form throughout the liquid and then rise out of the liquid (right). Temp • Stays the same until of a all of the substance substance has changed during phase• Why? change • Bc the energy is needed to change the substance, not the temperature When water reaches its boiling point, it stays at 100°C until it all changes to steam. Them temperature of the steam can then rise above 100°C. PHASE CHANGE GRAPH FOR WATER Condensation Boiling pt = 100°C Evaporation 100°C T E M P Freezing 0°C Melting Freezing/melting point = 0°C = phase changes Time What is a • 2 or more substances put together mixture? • No new substances • (not chemically combined) • Ex: salt water, salad… • Can be separated • (Boiling, magnets, Filtering…) What is a • Type of mixture solution? • 1 substance dissolved in another • 2 parts: • 1. Solute = what gets dissolved • 2. Solvent = what does the dissolving • Example: Hot chocolate • Solvent = Water • Solute = Chocolate Soluble • Able to dissolve • Ex: sugar is soluble in water What • 1. Heat affects • (more heat dissolves faster) the • 2. Surface area rate of dissolving? • (more surface area/smaller pieces dissolves faster) • 3. Stirring Solubility • Max amount of substance that will dissolve in a certain amount of liquid At 20°C, 38 grams of salt will dissolve in 100 grams of water. If more salt is added, it will not dissolve. It will sink to the bottom. Greatest solubility How does • Temperature temp increases solubility affect solubility? Solubility curves, like the one shown here, tell us what mass of solute will dissolve in 100g of water.