Functional Groups Notes - Study Package
advertisement

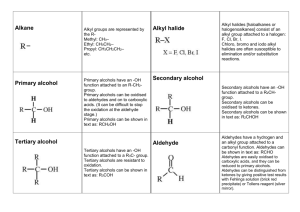
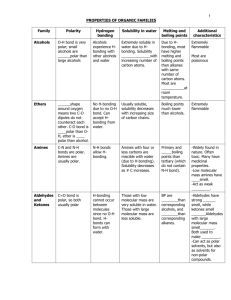
FUNCTIONAL GROUPS NOTE Alcohols • Organic compounds containing a hydroxyl group -OH • E.g. ethanol, cholesterol, retinol (vitamin A) Naming: • -ol suffix e.g. methane + OH = methanol Polyalcohols • Alcohols containing more than one -OH group • -diol, -triol suffix • Or hydroxy prefix 1,2-dihydroxyethane 1,2,3-trihydroxypropane Properties of Alcohols • More polar and can hydrogen bond – Higher boiling points – More soluble in polar solvents • Long-chain alcohols are nonpolar (hydrocarbon portion) and polar (-OH) – Ideal solvents in organic reactions because they will dissolve both polar and nonpolar compounds Hydration Reactions • Alkene + water --> alcohol 1 Combustion of Alcohols Elimination Reactions Dehydration (Condensation) Reactions: Under certain conditions alcohols can decompose to produce alkenes and water A catalyst (sulfuric acid) removes a hydrogen atom and a hydroxyl group from neighbouring carbons Resulting in C=C and H2O Ethers Molecules with C-O-C group More polar than hydrocarbons But, unlike alcohols, ethers cannot hydrogen bond Naming: • Add oxy to the prefix of the smaller hydrocarbon group and join it to the alkane name of the larger hydrocarbon group • E.g. CH3-O-C2H5 is methoxyethane 2 Condensation Reactions • When two alcohols combine, an ether and water are formed Aldehydes and Ketones Ketone: Molecule with a carbonyl group (C=O) between two carbon atoms. Alkane name with -one suffix Aldehyde: Molecule with a carbonyl group (C=O) on a terminal carbon. Alkane name with -al suffix Properties of Aldehydes and Ketones Lower boiling point and less soluble in water than alcohols (no -OH) More polar than hydrocarbons (higher boiling points and more soluble) Good solvents (both polar and nonpolar) 3 Oxidation Reactions Alcohol + an oxidizing agent (removes electrons) to form an aldehyde or ketone and water The oxidizing agent removes two H-atoms (one from the -OH group and one from the adjacent carbon) resulting in C=O and H2O Hydrogenation Reactions • The C=O double bond can undergo an addition reaction with hydrogen to form an -OH group. 4 Aldehydes always produce 1° alcohols • Ketones always produce 2° alcohols Carboxylic Acids • Molecules with a carboxyl group –COOH • E.g. lactic acid, citric acid Alkane name with -oic acid E.g. methanoic acid Properties of Carboxylic Acids Polar and can hydrogen bond Similar properties to alcohols (smaller members are soluble in water, larger members are insoluble) 5 pH < 7 (H-atom in -OH group) Oxidation Reactions Recall: A 1° alcohol can be oxidized to form an aldehyde. An aldehyde can be further oxidized to form a carboxylic acid A ketone cannot be oxidized because there is no free H-atom Esterfication Recall: acid + base --> salt + water (neutralization reaction) Carboxylic acid + alcohol --> ester + water Properties of Esters 6 • Similar to carboxylic acids, but lacking -OH group • Esters are less polar (less soluble in water), lower boiling points (no H-bonds) Not acidic Naming Esters • 1st part is the name of the alkyl group in the alcohol • 2nd part is the ending of the acid name changed from -oic to –oate Amines • An ammonia molecule in which one or more H-atoms are substituted by alkyl or aromatic groups Naming: • Amino + alkane name OR • Alkyl group + amine 7 Naming 2° and 3° Amines • N- prefix used for the substituted groups on the nitrogen atom • Alkyl groups are listed alphabetically Properties of Amines • N-C, and N-H polar bonds • H-bonding occurs but N-H is less polar than O-H Synthesizing Amines from Alkyl Halides Alkyl halide + ammonia --> 1° amine + HX 8 Alkyl halide + 1° amine --> 2° amine + HX Alkyl halide + 2° amine --> 3° amine + HX Amides • Similar to esters, except N instead of O Naming Amides • 1st part is the name is from the amine • 2nd part is the ending of the acid name changed from –oic to -amide • Alphabetical order with N- groups 9 N-ethyl propanamide Carboxylic acids + ammonia/1°/2° amines--> amides + water 10