Chapter 14
advertisement

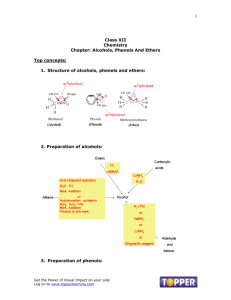
Chapter 14 - Organic Compounds containing Oxygen, Halogen or Sulfur Alcohols, Ethers, Alkyl halides & Thiols ROH RX ROR RSH All of these compounds contain a carbon atom that is singly bonded to a heteroatom! Alcohols & ethers are organic derivatives of water - Replacing H with one or two alkyl groups Alcohols Structural Characteristics R-OH -OH (hydroxyl = functional group) -OH is bonded to a saturated C atom! Classification Primary (1˚) C-C-OH Secondary (2˚) C-C-OH | C Tertiary (3˚) C | C-C-OH | C Alcohol Nomenclature IUPAC 1.Name the longest chain (drop the “e” and add “ol” at the end) to which OH is attached. 2.# the chain from the end nearest the -OH (# the position of the -OH group). 3.Name/locate any substituents. 4.For rings, -OH is on C#1. IUPAC naming examples Ex.: CH3OH Methanol CH3CHCH3 | 2-propanol OH 3,4-dimethylcyclohexanol •Alcohols with >1 -OH groups Ex.: CH2CH2 | | OH OH 1,2-ethanediol Alcohol Nomenclature Common (name “R” as an alkyl group) Alkyl group name + alcohol Ex.: CH3OH Methyl alcohol CH3CHCH3 | Isopropyl alcohol OH •Alcohols with >1 -OH groups •Ex.: Important Common Alcohols IUPAC Methanol Ethanol 2-propanol 1,2-ethanediol 1,2-propanediol 1,2,3-propanetriol Constitutional Isomerism Positional Ex.: butanol •Skeletal •Ex.: butanol Common •Methyl alcohol •Ethyl alcohol •Isopropyl alcohol •Ethylene glycol •Propylene glycol •Glycerol (glycerin) 1-butanol 2-butanol 2-butanol sec-butanol 2-methyl-2-propanol tert-butanol Physical Properties of Alcohols Alcohols have both Polar (-OH) & Nonpolar (alkyl) character! Properties are determined by which portion dominates - Short chain - polar end dominates: The polar hydroxyl functional group dominates the physical properties of methanol. - Long chain - nonpolar end dominates: Conversely, the nonpolar portion of 1- octanol dominates its physical properties. BP increases with increasing # of C atoms of London Dispersion Forces Water solubility Short chain - soluble Long chain - insoluble Alcohols can Hydrogen bond (better with small chain alcohols) Alkanes cannot Hydrogen bond Effect Chemical Reactions of Alcohols Combustion CH3OH + O2 --> CO2 + H2O Intramolecular Alcohol dehydration Conditions: 180˚C and H2SO4 catalyst Result: formation of alkene Ex.: C-C-OH ----> C=C + H2O Intermolecular Alcohol dehydration Conditions: 140˚C Result: formation of ether (R-O-R) Ex.: C-OH + HO-C ----> C-O-C + H2O Halogenation Reactions R-C-OH + X2 ----> R-C-X2 + H2O Oxidation Reactions Carbon atoms are oxidized if they lose H or gain O. Carbon atoms are reduced if they gain H or lose O. 1˚ alcohol ---> aldehyde ---> carboxylic acid 2˚ alcohol ---> ketone 3˚ alcohol ---> No Reaction! Preparation of Alcohols Alcohols can be prepared in two major ways: Alkene hydration Ex.: CH2=CH2 + H2O ----> CH3CH2OH Addition of H2 to a carbonyl group ( C=O) Ex.: Aldehyde + H2 ----> 1˚ alcohol Ketone + H2 ----> 2˚ alcohol Phenols Structural Characteristics -OH is attached to a C that is part of an aromatic ring. Ar-OH Nomenclature of Phenols Phenol = “phenyl” + “alcohol” IUPAC rules are same as for benzene derivatives. Parent ring is “phenol”. Physical & Chemical Properties of Phenols Flammable, like alcohols Phenols cannot be dehydrated. Oxidation occurs only with strong oxidizing agents. Halogenation Weak acids in solution (Ka~10-10) Occurrence & Uses of Phenols Antiseptics (but phenol derivatives are much safer than phenol itself). Mouthwashes, Lysol, etc. Antioxidant - several phenols are preferentially oxidized Food additives Vit. E Flavoring agents Irritants: poison ivy & poison oak Ethers Structural Characteristics Functional group = -C-O-CR-O-R R-O-R’ R-O-Ar Ar-O-Ar Nomenclature of Ethers IUPAC Select longest C chain = base name. Change -yl ending of other group to -oxy. (ie. Methyl becomes methoxy) Place alkoxy name (w/ locator #) in front of base chain name Common Name the two alkyl groups attached to the O and add the word “ether”. Isomerism Consitutional: partitioning of C atoms (by O) Isomers of individual alkyl groups Ex.: C4 ethers Functional Group Isomers: consitutional isomers with different functional groups Ex.: C3 ether and C3 alcohol Physical & Chemical Properties Physical BP = to alkanes; lower than alcohols No H-bonding w/ self possible Water soluble: can H-bond w/ water NP substances are generally soluble in ethers Chemical Flammable React w/ O2 to form unstable (explosive) ccompounds Unreactive w/ acids and oxidizing agents Halogenation Prepared by intermolcular dehydration of 1˚ alcohols Alkyl Halides: Incoming halogen atom (orange sphere) replaces a hydrogen atom in the alkane model. Naming: Treat halogen atoms like alkyl groups. F = fluoro; Cl = chloro; Br = bromo; I = iodo Ex.: CH3-CHBr-CHBr-CHI-CH2-CH3 Halogenation Reactions General equation: RH + X2 → RX + HX Hydrocarbon + Halogen Halogenated + acid (diatomic) hydrocarbon Ex. CH4 + Cl2 --> CH3Cl + HCl Highly exothermic reaction: can lead to an explosionThe process can continue to alter the resulting products as long as the halogen remains in sufficient quantities to drive further reactions. (The halogen would be the __________ reactant.) Chlorofluorocarbons (CFCs) Developed in the 1930's Very stable compounds composed of C, F, Cl, & H Freon is the tradename: Trichlorofluoromethane Dichlorodifluoromethane Trichloro-trifluoroethane Dichloro-tetrfluoroethane Chloropentafluoroethane The Ozone Layer Chemistry CFCl3 + UV Light ==> CFCl2 + Cl Cl + O3 ==> ClO + O2 ClO + O ==> Cl + O2 The chlorine free radical atom is then able to attack another ozone molecule Cl + O3 ==> ClO + O2 ClO + O ==> Cl + O2 and again ... Cl + O3 ==> ClO + O2 ClO + O ==> Cl + O2 and again... thousands of times! A catalyst! Thiols = Mercaptans: sulfhydryl group (-SH) bonded to a saturated C atom Alcohol - R-OH C-C-OH (ethanol) or (ethyl alcohol) Thiol - R-SH C-C-SH (ethanethiol) or (ethyl mercaptan) Properties of Thiols Physical Low BP: no H-bonding Strong odor: Skunks, Methanethiol (additive to natural gas), Morning breath Chemical Oxidation-Reduction 2 thiols <==> Disulfide Important in Protein chemistry Thioethers - replace the “O” with “S” (R-S-R) C-S-C C-S-C-C-C Ar-S-C C=C-S-C C=C-C-S-C C=C-C-S-S-C-C=C Morning Breath = Hydrogen sulfide. Methanethiol, Dimethyl sulfide… Onions Garlic What do you need to know? Structural characteristics (know the functional group) Alcohol Phenol Ether Alkyl halides Sulfur Analogs Isomers Nomenclature (the rules for naming the molecules) Common & IUPAC Physical properties (basic/simple) BP; Solubility; Flammability Occurrence and uses (common) Natural (ex.: menthol, skunk, nutmeg, clove, garlic, onion) Synthetic (ex.: antiseptics, racing fuel, de-icers, antioxidants, anesthetics) Preparation (what basic reactions produce the molecules) Alcohols - alkene hydration; Phenols - benzene hydration Ethers - intermolecular alcohol dehydration Characteristic reactions of the molecules Combustion; dehydration*; halogenation; oxidation