Alcohols - Miller, Jonathan
advertisement

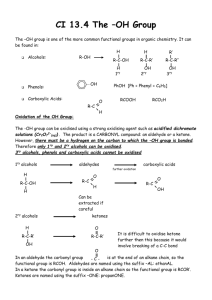
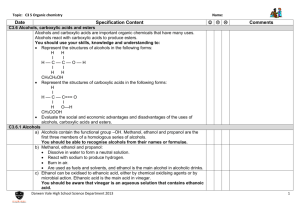
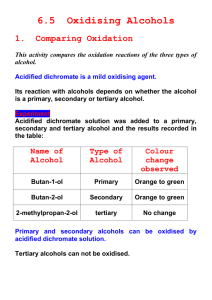
10.4 Hydroxy Compounds Alcohols Learning Outcomes (a) recall the chemistry of alcohols, exemplified by ethanol: (i) combustion (ii) substitution to give halogenoalkanes (iii) reaction with sodium (iv) oxidation to carbonyl compounds and carboxylic acids (v) dehydration to alkenes (vi) ester formation (b) (i) classify hydroxy compounds into primary, secondary and tertiary alcohols (ii) suggest characteristic distinguishing reactions e.g. mild oxidation Alcohols Primary alcohols Secondary alcohols Tertiary alcohols (i) Combustion C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) (ii) substitution to give halogenoalkanes Alcohols react with hydrogen halides (HCl, HBr, HI) to give the halogenoalkane 2KBr(s) + H2SO4(l) C2H5OH(l) + HBr(g) K2SO4(s) + 2HBr(g) acid catalyst C2H5Br(l) + H2O(l) With potassium iodide, phosphoric(V) acid is used, as conc. sulphuric reacts with KI to produce iodine. Mechanism of substitution SN2 mechanism - nucleophilic substitution: Primary Alcohols H H H H C C O H H fast H H H H H H H C C O H H X- + + H H H slow H H H C C O + H+ H C C X H + H2O H H SN1 mechanism - nucleophilic substitution: Tertiary Alcohols CH3 H3C C O + CH3 H H slow -H2O CH3 H3 C C + XCH3 carbocation fast CH3 H3C C X CH3 A tertiary alcohol will react with concentrated acids, e.g. shake with conc. HCl (iii) Reaction with sodium 2C2H5OH(l) + 2Na(s) C2H5O-Na+(s) + H2(g) sodium ethoxide Alkoxides are strongly basic and will remove a proton from water to form a hydroxide ion (pH 14), so they are not used in aqueous solution! C2H5O-Na+(s) + H20(l) C2H5OH (l) + Na(OH)(aq) Alkoxides are also good nucleophiles (S2N); ether formation: Ester formation (esterification) Esters are fragrant smelling liquids used in perfumes and fruit flavourings (often larger molecular masses) formed when an alcohol reacts with a carboxylic acid in the presence of an acid catalyst (small amount of conc. H2SO4). It is an equilibrium reaction. acid + alcohol ester ethyl ethanoate Esters are named with the alcohol side first (ethyl from ethanol) then the carboxylic acid (ethanoate from ethanoic acid). It is not necessary to understand the mechanism of their formation (catalytic addition to C=O/elimination of H2O). (iv) Oxidation to carbonyl compounds and carboxylic acids Primary alcohols can be oxidised to either aldehydes or carboxylic acids depending on the reaction conditions. In the case of the formation of carboxylic acids, the alcohol is first oxidised to an aldehyde which is then oxidised further to the acid. Note that the orange chromate(VI) is reduced to green Cr3+(aq). The Police use this procedure to detect alcohol levels in the breath of drunk drivers. (a) Partial oxidation to the aldehyde You get an aldehyde if you excess of the alcohol and distill off the aldehyde as soon as it forms. (b) Full oxidation to the carboxylic acid You need to use excess of the oxidizing agent and make sure the aldehyde formed half-way stays in the reaction mixture. Again we see a colour change in the chromate. Secondary alcohols Secondary alcohols are oxidized to ketones; there is no further oxidation to the carboxylic acid For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate(VI) solution acidified with dilute sulphuric acid, you get propanone formed. Tertiary alcohols can’t be oxidized in this way. We can used selective oxidation as distinguishing reactions between primary, secondary and tertiary alcohols. In summary: Dehydration to alkenes With longer chain or branched alcohols, a mixture of alkenes may be produced including cis and trans isomers, at around 600 K. Aluminium oxide is often used (or a ceramic like pumice). Concentrated sulphuric and phosphoric (V) acids are alternative dehydrating agents when boiled.