Regents Unit 15: Hydrocarbon Derivatives
advertisement

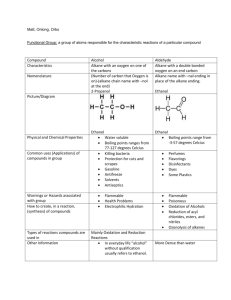
•Atom or group of atoms that replaces a hydrogen atom in a hydrocarbon. •Functional groups give the molecule personality. Functional Group •Each functional group gives the molecule distinctive chemical & physical properties. •Molecules with functional groups contain at least one atom that is not C or H. Not hydrocarbons! Functional Group •Derived from the name of the hydrocarbon with the same number of carbon atoms. Names of molecules with functional group •One or more H in an alkane is replaced with a halogen (F, Cl, Br, or I). •General formula = R-X. –X is the halogen. –R is the alkyl branch. Halides or halocarbons or alkyl halides •Likely to be polar molecules, unless very symmetric. •Dipole-dipole interactions. Properties of Halides •Have POLES. •The electron cloud is lop-sided. One end of molecule is electron-rich & the other electron-poor. •Dipole-dipole interactions. Polar Molecules •Atoms in molecule have very different electronegativities. •Molecule is not symmetrically shaped. Polar Molecules •Higher mp, bp, Hf, Hv than the corresponding alkanes. •Lower rate of evaporation & vapor pressure than the corresponding alkanes. Properties of Halides •One or more hydrogens in a hydrocarbon replaced with an OH group. •General formula = R-OH. •The OH or hydroxyl group does NOT ionize in water. Alcohols •NONelectrolytes. No H+ or OH- ions in solution. •Do NOT turn litmus red, etc. Properties of Alcohols •Contain O-H bond. Molecules tend to be polar. Dissolve in water. •Hydrogen bonding takes place between alcohol molecules. Higher mp, bp than corresponding alkanes. Properties of Alcohols •Replace final –e of corresponding alkane name with –ol. Naming Alcohols •Monohydroxy: 1 -OH group. •Dihydroxy: 2 –OH groups. •Trihydroxy: 3 -OH groups. Classification of alcohols by number of -OH groups. •Primary: OH group at end carbon. •Secondary: OH group on middle carbon. •Tertiary: OH group at branch-point carbon. Further classification of monohydroxy alcohols by carbon to which OH group attached. •Contain an O atom bridge connecting 2 alkyl branches. •General Formula: ROR‘ •R & R‘ are alkyl groups (the 2 branches). •R & R‘ can be same or different. Ethers Tend to be nonpolar. Properties of Ethers 1. Name branches alphabetically. 2. Stick the word “ether” at the end. Naming Ethers C in a chain that has a double bond to an O. >C=O O or C Carbonyl Group Contain a carbonyl group at the end of the chain. Aldehydes Replace final “e” of corresponding alkane name with “al.” Naming Aldehydes Contain a carbonyl group on a carbon atom inside the chain, not at the end. Ketones Replace final “e” of corresponding alkane name with “one.” Naming Ketones Carbonyl group is quite polar. Tend to be soluble in both polar & nonpolar solvents. Properties of ketones Contain a –COOH group at the end of the molecule. O -C-O-H Organic Acids The H in the –COOH group is acidic! O -C-O-H Organic Acids Turn litmus red. Electrolytes! O -C-O-H Properties of organic acids Replace the final –e of the corresponding alkane name with “-oic acid” Naming organic acids Contain 2 oxygens! 1) Bridge O in the middle of the chain. 2) Carbonyl O right next door. Esters R-CO-OR‘ Bridge O Carbonyl O O R-C-O-R' General formula of esters 1. Name alkyl branch next to bridge O. 2. Name branch with carbonyl group. 3. Replace –e of corresponding alkane name with -oate. Naming Esters Format of esterification rxn: Alcohol + Org. Acid Ester + H2O Formation of Esters HO H H H HCCOCCCH + H2O H H H H H O HHH HCCOH + HOCCCH HHH H Formation of Esters 1. Replace H in an alkane with – NH2 group. 2. Replace final –e in alkane name with amine. 3. # gives location of NH2 group. 4. Analogous to alcohols Amines 1. Tend to smell really bad. 2. Contain N-H bond. Exhibit Hbonding between molecules. Properties of Amines 1. Contain NH2 group bonded to a carbonyl group. O 2. General formula: R-C-NH2 Amides 1. Find name of corresponding alkane. 2. Drop final -e & add –amide. 3. Never need a # - Always at the end. Naming Amides 1. Contain COOH group at end. 2. C right next door has NH2 or amino group. Amino Acids 1. Large molecules made of chains of smaller units covalently bonded together. Polymer 1. Each individual unit of a polymer Monomer 1. Reaction leading to formation of polymers Polymerization