CHAPTER 1: ORGANIC COMPOUNDS
advertisement

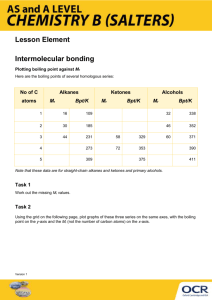
CHAPTER 1: ORGANIC COMPOUNDS NOTE: I highly recommend that you do the practice questions to help you learn. If you can’t explain it, you don’t know it! Key Ideas: see p 6. Read Introduction. Organic compounds: carbon is the principle element 1.1: Functional Groups. - active areas of the molecule - determine the physical and chemical properties - each organic family has its own functional group - 3 components: 1. ________________________________________________________________ 2. ________________________________________________________________ 3. ________________________________________________________________ Carbon-Carbon multiple bonds: - single bonds C-C are strong - a second or third bond is less strong C=C or CC so a reaction that breaks the second or the second and third is likely Single Bonds between C and a more electronegative atom: - ex: O, N, F, Cl, Br - bond is polar, so attraction among molecules increases, so mp and bp go up - if C-O-H or C-N-H, the H is very positive so very polar so even higher mp and bp and ability to dissolve in polar solvents like water (like dissolves like) Double-Bonded C and O - C=O means the bond is more polar than C-O so increase in mp and bp and more ability to dissolve in polar solvents 1.2: Hydrocarbons: Aliphatic hydrocarbons: ______________________________________________________________ - alkanes: _______________________________________________________________________ - alkenes: _______________________________________________________________________ - alkynes: ________________________________________________________________________ Aromatic hydrocarbons: _______________________________________________________________ see p 11 table 1 Nomenclature of Hydrocarbons: ( IUPAC ) Alkanes: suffix “-ane” branch chain: suffix: “-yl” - know forst 10 prefixes – see p 12 table 2 Isomers - __________________________________________________________________________ - see Summary of naming, p 15 Alkenes and Alkynes: suffixes “-ene” and “-yne” - see summary p 18 Aromatic Hydrocarbons: - benzene is the parent, alkyl groups atached - if the side chain isn’t attached to the benzene ring on the end C, then the chain may be the parent and the benzene ring the attachment – called the phenyl group - see summary p 21 Physical Properties of Hydrocarbons: 1. low mp and bp – 2. the smaller the molecule, the lower the mp and bp 3. non-polar – C and H have almost the same electronegativities and so form non-polar bonds 4. make good non-polar solvents 5. don’t dissolve in polar solvents such as water 1.3: Reactions of Hydrocarbons - all burn to give CO2, H2O and huge amounts of heat energy - less reactive - Alkanes aromatic compounds alkenes alkynes – more reactive Reactions of Alkanes: - single C-C bonds stable and so unreactive, generally - combustion - Substitution reactions – add halogen gas, one H is replaced with one halogen alkyl halide + acid and if excess halogen gas is present, further substitutions can happen Reactions of Alkenes and Alkynes: - Addition reactions: the double / triple bond is broken and an atom is added to each C : 1. add halogen get alkyl compound with 2 halogens ( halogenation ) 2. Hydrogenation: add hydrogen gas, get alkane - unsaturated fats – have alkyl groups with many double bonds – lower mp - saturated fats – hydrogenation reactions – get fats with higher mp 3. add hydrogen halide and get alkane with single halide 4. Hydration: add water, get alkane with OH group – an alcohol - Markovnikov’s Rule: “ the rich get richer” – if addition reaction with non-identical atoms being added, the H gets added to the C that already has more H’s attached to it Reactions of Aromatic Compounds: - Substitution reactions: H replaced with another group - due to nature of bonds in benzene ring: e- shared equally by all 6 C’s, so bonds between C’s are stronger and shorter than single C-C bonds but longer and less strong than C=C bonds. - typical “resonance” structure - see Summare p 30 1.4: Organic Halides - one or more H’s replaced with a halogen atom - naming – see p 32 Properties of Organic Halides - Halogens have higher electronegativity than H so C-X bonds more polar - so substances have higher mp and bp - and more able to dissolve in polar solvents than alkanes - molecules with 2, 3 or more halogen atoms may be formed when halogen gas is added to alkanes - the greater the number of halogen atoms, the more polar the molecule, the higher the mp and bp, so they can be separated - see table 1 p 34 The Cost of Air Conditioning - refrigerants – CFC’s such as CF2Cl2 - stable , but decompose in upper atmosphere under UV light - release Cl atom – reacts with ozone to form O2 - one Cl atom can decompose 1 000 000 molecules of ozone - new compounds now used: HCCl’s and HCF’s – presence of H causes less stable compound when released into atmosphere - these decompose more quickly and at lower atmosphere levels so less ozone is affected - drawback: CO2 produced when HCF’s decompose – adding to greenhouse gases Preparing Organic Halides – addition reaction to alkenes or alkynes - substitution reaction with benzene Preparing Alkenes from Alkyl Halides - Elimination Reaction: alkyl halide in a base ( hydroxide ion ) alkene + water + halide ion - see summary p 36 1.5: Alcohols and Ethers Alcohols: - contain OH group – hydroxl group - ex: ethanol – alcohol in drinks, poisonous, depressant see p 38: Alcohol toxicity - retinol – Vitamin A Naming Alcohols - end in “-ol” - or: OH group is considered to be added to the parent chain : 2-hydroxybutane instead of 2-butanol 1o, 2o, 3o Alcohols: - primary – OH is on an end C – most reactive kind - secondary – OH is attached to a C that is attached to 1 other C - tertiary – OH is attached to C that is attached to 2 other C’s Polyalcohols: - 2 or more OH groups - use alkane plus “-diol” or “-triol” … - ex: antifreeze – commonly called ethylene glycol – 1,2-ethanediol, it’s soluble in water, sweet-tasting ad poisonous – wash away spills outside – cats and dogs die! - ex: glycerol ( glycerine) 1,2,3-propanetriol, non-toxic, very soluble in water, used in lotions and chocolate - ex: sugars have OH groups Cyclic Alcohols: - based on cycloalkanes: ex menthol and chlosterol - based on benzene: ex phenol - use hydroxyl prefix for naming Properties of Alcohols - bp higher than parent alkanes – due to polarity - smaller ones much more soluble in water than parent alkanes – due to polarity - if alkyl chain is long, then non-polar substances will dissolve in the alcohol Reactions involving Alcohols: - preparation: hydration of an alkene - Combustion: form CO2 and water - from alcohol to alkene: Elimination reaction with conc. H2SO4 ( dehydration ) summary – see p 45 Ethers: - first used as an anesthetic Properties of Ethers: - two alkyl groups attached to an oxygen - alkyl groups may be the same or different - C-O-C bond is v-shaped and polar, so molecule is more polar than an alkane with the same number of C’s but not as polar as alcohols with the O-H bond - see table 2 p 46 - can dissolve both polar and non-polar substances - C-O bond stable so they are unrreactive Naming Ethers: - use “-oxy” on end of shorter alkyl group as prefix to larger alkyl group - ex: ethoxybutane for: CH3CH2-O-CH2CH2CH2CH3 - or name the two alkyl groups: ethyl butyl ether * three separate words this time Preparing Ethers from Alcohols: - Condensation reaction: 1 water molecule eliminated when 2 alcohol molecules combins use conc. sulfuric acid as the catalyst 1.6: Aldehydes and Ketones: - C=O gp called carbonyl gp - Aldehyde: carbonyl gp on end C - small aldehyde molecules smelly ex: formaldehyle and acetaldehyle stink and are preservatives and disinfectants - larger ones – flower smells ex: iol of almonds - benzaldehyhde - Ketone: carbonyl gp not on end C - insects can smell them from far away - ex: ants follow trails, pheromones attract insect mates - ex: propanone (acetone) is a good solvent Naming Aldehydes and ketones: - “-al” and “-one” - number the C the =O is on for ketones Properties of Aldehydes and ketones: - lower mp and bp than alcohols but higher than the parent alkanes - the C=O bond is polar - can dissolve in both polar and non-polar substances so make good solvents Preparing Aldehydes and Ketones from Alcohols: Oxidation Reactions - oxidation reaction: _________________________________________________________________ - controlled oxidation reaction: H removed from the molecule and combines with oxygen to form water - Oxygen supplied by oxidation reagents ex: KmnO4 and K2CR2O7, with conc sulfuric acid as a catalyst - primary alcohols make aldehydes - secondary alcohols make ketones - tertiary alcohols don’t react – the C with the OH group doesn’t have aaa H to contribute - see summary p 53 From Aldehydes and Ketones to Alcohols: Hydrogenation Reaction: - add hydrogen to cause C=O bond to break and 2 H atoms added to make an alcohol - C=O bond is strong – hydrogenatiiion needs high temp and pressure and a catalyst - aldehydes make primary alcohols - ketones make secondary alcohols see summary p 55. 1.7: Carboxylic Acids and Esters Carboxylic Acids: - weak acids - ex: vinegar – when alcohol made by fermenting food is further oxidized - ex: lactic acid – sour milk, and causes muscle pain when a build-up in the muscles after lots of exercise Naming Carboxylic acids: - carboxyl group: COOH, combines hydroxyl group OH, and carbonyl group C=O - “-oic acid “ - carboxyl group on either end of the alkane or alkene chain: “-dioic acid” - ex: methanoic acid (formic acid) – in and ant bites bee stings, removes hair from hides - ex: ethanoic acid ( vinegar, acetic acid), cooking, dying, solvent - ex: phenylmethanoic acid (benzoic acid) – simplest aromatic acid – to make sodium benzoate to preserve foods - ex: oxalic acid, tartaric acid, citric acid, Vitamin C, acetylsalicylic acid, ….. Properties of Carboxylic Acids: - carboxyl group is polar - so mp and bp are higher than for parent alkanes - all properties of non-organic acids – litmus pink, neutralize bases… - react with organic “bases” (alcohols) to form organic “salts” (esters) Preparing Carboxylic Acids: - controlled oxidation of an alcohol to an aldehyde and then to a carboxylic acid - ex: use potassium dichromate as a source of oxygen and the Cr ion goes from +6 to +6 and from orange to green – the breathalyzer test! - see summary p 62 From Carboxylic Acids to Organic “Salts”: Esterification - carboxylic acid + alcohol, with conc sulfuric acid and heat, gives an ester – an organic salt Esters: give odours to fruits and flowers see table p 64 Naming and Preparing Esters: - are salts - alkyl group from the alcohol + alkyl group from the acid”-oate” 2 words - Condensation reaction: acid + alcohol ester + water Properties of Esters: - don’t have the OH group so less polar than parent acids - less soluble in water and lower mp and bp than parent acids - no acidic properties - smaller ones gases at room temp and so we can smell them - larger ones are waxy Reactions of Esters: Hydrolysis - use strong base to break C-O bond and get back the acidic ion and alcohol - fats are esters and when cooked with strong base becone the sodium salts of the fatty acids – soap - summary: see p 67 1.8: Amines and Amides: Amines: - from NH3 where 1, 2, or 3 H’s are replaced by alkyl groups - from breakdown of proteins by bacteria – smell bad - 1o – primary – one alkyl group in place of one H on ammonia molecule - 2o- secondary – 2 alkyl groups in place of 2 H’s on ammonia molecule - 3o – tertiary – 3 alkyl groups Naming Amines: - “amino” prefix to the alkane ex: cadaverine is 1,5-diaminopentane - or alkyl prefix to the “amine” Properties of Amines: - N-C bond and N-H bond are polar so amines have higher mp and bp, but lower than for alcohols because N-C nad N-H bonds are less polar than O-C and O-H bonds - dissolve in water - small ones are gases at room temp and smell “fishy” Preparing Amines: - ammonia + alkyl halide – primary amine - excess alkyl halide can lead to more substitutions to get secondary and tertiary amines as well - see summary p 73 Amides: - similar to esters – O is replaced by N - amide bonds called peptide bonds in proteins – hold proteins together Naming and Preparing Amides: - Condensation reaction: acid + (ammonia or 1o or 2o amine) amide + water - alkyl from the amine + alkyl from the acid”-amide” 2 words - see summare p 75 Properties of Amides: - weak bases - insoluble in water ( except smaller ones slightly soluble due to N-H bond ) - N bonded to 2 H’s higher mp and bp – polar bonds Reactions of Amides: - hydrolysis with bases ( or acids) to reform acids and amines - happens less spontaneously than for esters - good because means proteins hold together quite well – stable - see summary p 78. 1.9: Synthesizing Organic Compounds: - more kinds made daily to experiment or to fulfill an exact need - read the rest for interest. SUMMARY p 83 !! SUMMARY p 92 – 93 Problems you can solve p 94 – to help you study Self-Quiz: do it! answers in the back of the book Problem Set: p 96 1, 2, 3 odds, 4, 5 odds, 6, 7 odds, 8 odds, 9, 10 odds, 11, 14, 15a,b,d. (You should be able to do all parts of the questions where I required only the “odds”. ) Labs: 1.3.1 dry, 1.5.2 short, 1.7.2 full lab report, 1.9.1 short.