G.G.N PUBLIC SCHOOL Assignment- Chemical Bonding CLASS
advertisement

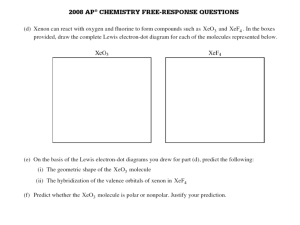
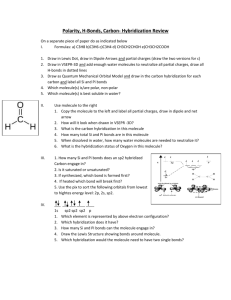
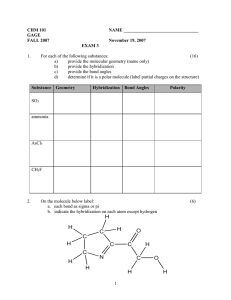
G.G.N PUBLIC SCHOOL Assignment- Chemical Bonding CLASS- XI 1 Use molecular orbital theory to explain why Be2 molecule does not exist. 2 Explain the formation of and bonds in C2H4 with the help of Diagram. Mention the hybrid state of two carbon atoms. 3 What is the hybridization of central atom in following-- NH3 , C 2 H2 4 What is the dipole moment of CCl4 molecule? Account for your answer. 5. Describe the hybridization in case of PCl5. Why are axial bonds longer as compared to the equatorial bonds? 6 Which of the two as higher dipole moment and why? NF3 or NH3 7 Which of the two is steam volatile and why? o-nitrophenol or p-nitrophenol . 8 What shapes are associated with sp3d and sp3d2 hybrid orbitals? Explain with one eg of each. 9 .In the reaction: N2 N2+, O2 ---O2+, Why is there an increase in bond order in going from O2 ---O2+, while there is a decreaseon going from N2 N2+ ? 10 All C-O bond lengths in CO3 2-are equal. Account for this observation. 11 Explain why BeH2 molecule has zero dipole moment although the Be- H bonds are polar? 12 Draw the resonating structures of carbon dioxide molecule. 13 Why is NF3 trigonal pyramidal while BF3 is trigonal planar, though both are tetra atomic molecules? 14 Predict the shape of ClF3 and BF3 on the basis of VSEPR theory 15 Draw lewis structure of a) Phospine b) Hydrogen peroxide c) Nitrogen trifluoride 16 a) Compare dipole moment of Water, Ammonia and Nitrogen trifluoride b) Why water has bent shape.