Polarity, H-Bonds, Carbon
advertisement

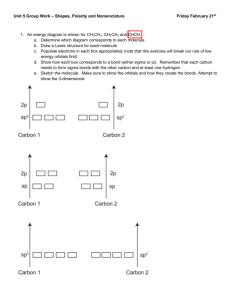
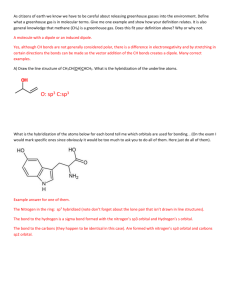
Polarity, H-Bonds, Carbon- Hybridization Review On a separate piece of paper do as indicated below I. Formulas: a) C3H8 b)C3H6 c)C3H4 d) CH3CH2CHOH e)CH3CH2COOH 1. Draw in Lewis Dot, draw in Dipole Arrows and partial charges (draw the two versions for c) 2. Draw in VSEPR-3D and add enough water molecules to neutralize all partial charges, draw all H-bonds in dotted lines 3. Draw as Quantum Mechanical Orbital Model and draw in the carbon hybridization for each carbon and label all Si and Pi bonds 4. Which molecule(s) is/are polar, non-polar 5. Which molecule(s) is best soluble in water? II. Use molecule to the right 1. Copy the molecule to the left and label all partial charges, draw in dipole and net arrow 2. How will it look when drawn in VSEPR -3D? 3. What is the carbon hybridization in this molecule 4. How many total Si and Pi bonds are in this molecule 5. When dissolved in water, how many water molecules are needed to neutralize it? 6. What is the hybridization status of Oxygen in this molecule? III. 1. How many Si and Pi bonds does an sp2 hybridized Carbon engage in? 2. Is it saturated or unsaturated? 3. If synthesized, which bond is formed first? 4. If heated which bond will break first? 5. Use the pix to sort the following orbitals from lowest to hightes energy level: 2p, 2s, sp2. IV. ___ ___ ___ ____ ____ 1s sp2 sp2 sp2 p 1. Which element is represented by above electron configuration? 2. Which hybridization does it have? 3. How many Si and Pi bonds can the molecule engage in? 4. Draw the Lewis Structure showing bonds around molecule. 5. Which hybridization would the molecule need to have two single bonds?