1. For each of the following substances: (16)
advertisement
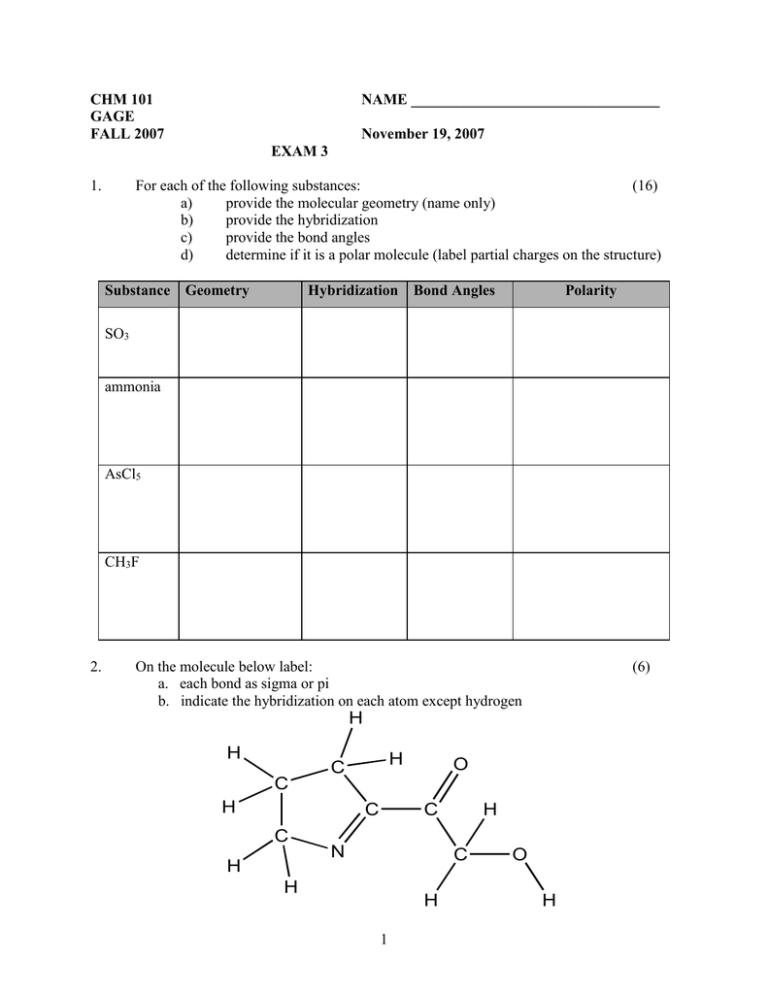
CHM 101 GAGE FALL 2007 NAME _________________________________ November 19, 2007 EXAM 3 1. For each of the following substances: (16) a) provide the molecular geometry (name only) b) provide the hybridization c) provide the bond angles d) determine if it is a polar molecule (label partial charges on the structure) Substance Geometry Hybridization Bond Angles Polarity SO3 ammonia AsCl5 CH3F 2. On the molecule below label: a. each bond as sigma or pi b. indicate the hybridization on each atom except hydrogen (6) H H H C O C H C C H C N H C H H 1 O H 3. Rank the following molecules with the dipole moments listed from most (1) to least (5) polar. For each molecule, draw the structure and show the direction of the molecular dipole using the appropriate symbol. (10) Compound Dipole Moment Rank (1, 2…) Structure with molecular dipole 4. SiH3F 1.27 AsH3 0.20 OF2 0.297 NH3 1.47 AsCl3 1.59 A 200.0 g sample of water is heated from 20oC to 130oC. sp.htsteam = 2.10 J/g oC Hfus = 0.332 kJ/g sp.htliq water = 4.18 J/g oC Hvap = 2.26 kJ/g o sp.htice = 2.09 J/g C a. Is the overall change endothermic or exothermic? Explain. b. Explain what is happening on a molecular level at each step in the process. Include types of motions. (6) c. Determine the total amount of energy exchanged by the system. 2 (2) (12) 5. Use the boxes above to answer the questions below. The two sealed, rigid boxes have the same volume and are at the same temperature. (10) a. Are all the particles in the argon box moving with the same velocity? Explain. b. Do the particles in both boxes have the same average velocity? Explain. c. How do the pressures in the two boxes compare? Justify your answer. d. Explain how you could increase the pressure in the xenon box without opening it. e. In the box, draw the view of the contents of the argon box if the temperature was dropped to -30oC. (b.p. of argon= -186oC) 3 6. 7. For each of the pairs of materials listed below. Circle the substance with the lower value for the property listed and explain why it has the lower value. (8) a. boiling point CBr4 CCl4 b. melting point C5H12O2 Na2SO4 a. What is the temperature in oC of 19.5 g of nitrogen gas at 1100 mmHg in a 47.0L container? (10) b. If you have 235 L sample of carbon dioxide at STP, what will its pressure be at a volume of 440L and 100oC? (10) 8. Fill-in: Select the most appropriate word, phrase or value for each statement below: (10) a. crystal with high melting point and no conductivity a. ____________________________ under any conditions b. intermolecular force in a molecular crystal b. ____________________________ c. example of a covalent solid c. ____________________________ d. tendency for liquid surface to contract d. ___________________________ e. factor that affects boiling point but not melting point e. ___________________________ 4




