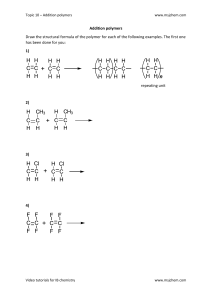
1. Estimate the magnitude and orientation of the electric dipole moment of the planar amide group shown in (5) by using the partial charges (as multiples of e) and the locations of the atoms Shown as (x,y,z) coordinates with distances in picometres. Please note that the partial charges are multiples of the fundamental charge e = 1.602 × 10−19C 2. Estimate the magnitude of the electric dipole moment of methanal(formaldehyde) by using the information above. 3. 4. 5. 6. What is a difference between polarization and polarizability? Give three characteristics of polarizability How polarizability relates with electric field? Explain the below diagram using N2 as an example 7. Use the below diagram to explain an exception of tetrahedral, octahedral, and icosahedral molecule when subjected to an electric field. 8.lllllll 9. Explain the below diagram 10. The relative permittivity of camphor(8) was analysed with a series of temperatures with results given in the diagram below. Determine the dipole moment and polarizability volume the molecule. Hint: 11. What is a difference between monodispersed and polydisperse molecule? 12. 13. Give 3 differences of primary, secondary, tertiary, and quaternary structures of macromolecules 14. Explain the below diagram by 3D coil and radius of gyration 15. Explain the below diagram using plastic and a rubber as an example 16. Why it an importance to measure mechanical properties of a material? 17. What is a difference between tough and strong materials? 18. Is a plastic verb or a material? Explain by the help of the below diagram. 19. How thermal properties could assist to identify a polymer? 20. What is a difference between phase change and thermal degradation of polymers? 21. Define the following temperature event of polymers a) Melting temperature b) Glass transition c) Cooling temperature d) Cross linking 22. Mention and discuss 5 factors affecting Glass transition of polymers 23. Explain the below diagram