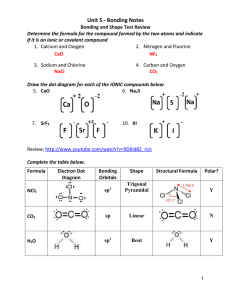
MC Review
advertisement

2003 (A) CO2 (B) H2O (C) CH4 (D) C2H4 (E) PH3 3. The molecule with only one double bond. 4. The molecule with the largest dipole moment 5. The molecule that has trigonal pyramidal geometry • 3)D • 4)B • 5)E • In which of the following groups are the three species isoelectronic? a) b) c) d) e) S2-, K+, Ca2+ Sc, Ti, V2+ O2-, S2-, ClMg2+, Ca2+, Sr2+ Cs, Ba2+, La3+ • A Of the following compounds, which is the most ionic? (a) SiCl4 (b) BrCl (c) PCl3 (d) Cl2O (e) CaCl2 • E • Which of the following oxides is gas at 25 degrees C? (a) Rb2O (b) N2O (c) Na2O2 (d) SiO2 (e) La2O3 • B A pure, white crystalline solid dissolves in water to yield a basic solution that liberates a gas when excess acid is added to it. On the basis of this information, the solid could be (a) KNO3 (b) K2CO3 (c) KOH (d) KHSO4 (e) KCL • B Types of Hybridization exhibited by the C atoms in propene, CH3CHCH2 include which of the following? I. sp II. sp2 III. Sp3 (a) (b) (c) (d) (e) I only III only I and II only II and III only I, II, and III • D • Of the following molecules, which has the largest dipole moment? (a) CO (b) CO2 (c) O2 (d) HF (e) F2 • D The electron-dot structure (Lewis Structure) for which of the following molecules would have two unshared pairs of electrons on the central atom? (A) H2S (B) NH3 (C) CH4 (D) HCN (E) CO2 • A I2(g) + 3Cl2(g) 2ICl3(g) According to the data in the table below, what is the value of DHo for the reaction represented above? Bond Average Bond Energy kJ/mole I–I 149 Cl – Cl 239 I – Cl 208 (a) (b) (c) (d) (e) -860 kJ -382 kJ +180 kJ +450 kJ +1258 kJ • B Which of the following molecules has a dipole moment of zero? (a) C6H6 (b) NO (c) SO2 (d) NH3 (e) H2S • A Molecules that have planar configuration include which of the following? I. BCl3 II. CHCl3 (a) (b) (c) (d) (e) I only III only I and II only II and III only I, II and III only III. NCl3 • A The SbCl5 molecule has a trigonal bipyramidal structure. Therefore the hybridization of Sb orbitals should be. a. b. c. d. e. sp2 sp3 dsp2 dsp3 d2sp3 • D CCl4, CO2, PCl5, PCl3, SF6 • Which of the following does not describe any of the molecules above? (a) Linear (b) Octahedral (c) Square planar (d) Tetrahedral (e) Trigonal pyramidal • C