Co-ordinate Bonds, Intermolecular Forces and Metallic Bonding
advertisement

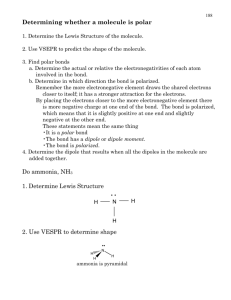
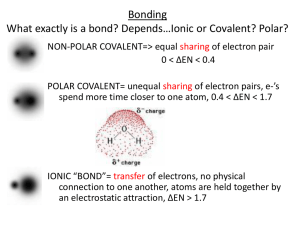
Co-ordinate Bonds, Intermolecular Forces and Metallic Bonding Everything you EVER wanted to know but were afraid to ask! Co-ordinate Bonding Also referred to as DATIVE bonding. Occurs when a PAIR of electrons is donated from one atom to another. Only happens when an empty orbital is present (ie an electron deficient species) Once the coordinate bond is formed, we treat it exactly the same as a normal covalent bond. This is how Group 13 atoms get full octets! Amino-Borane Huh!?!?!? NH3 – BH3 (don’t worry about the name) Draw the Lewis Structure for NH3, then for BH3 N can “donate” its lone pair to B forming a co-ordinate bond. N has a full octet an a lone pair of electrons. NH3 is an uncharged molecule. B only has 6 electrons, it is deficient. BH3 is an uncharged molecule. Amino-Borane Now we have a new molecule with a covalent bond between N and B. While there are formal charges on N and B, the overall charge on the molecule is neutral. The bond formed between N and B is just as strong as any covalent bond. Co-ordinate Bonding Draw the co-ordinate bonds between each of the following pairs: GaCl3, ClH+, H2O H+, NH3 BF3, NH3 NH3, GaCl3 PCl3, GaCl3 AlCl3, AlCl3 (to form Al2Cl6) Intermolecular Forces Covalent Bonds are NOT intermolecular forces. They are INTRAMOLECULAR forces. So what are INTERMOLECULEAR Forces? The forces of attraction/repulsion that exist between molecules. The ones we need to overcome to change the state of the substance. Relative Strengths of Bond Types Ionic Covalent Intermolecular Forces Van der Waals Forces Discovered but the Dutch physicist, Van der Waals and named in his honor. They are weak interactions between molecules are divided into 2 basic types: 1. Dipole – Dipole When a molecule has a permanent dipole moment (molecule is polar), the negative end of one molecule will sit closer to the positive end of its neighbor. 2. Dispersion or London Forces (weakest of all intermolecular) Caused by temporary shifts in electron density within a molecule. For instance the 2 e- shared in H2, located on 1 H atom rather than the other. Dipole – Dipole Forces d+ d- d+ d- d+ d+ d- d+ d- d- Ion – Dipole Forces Exist when we dissolve an ionic compound in a polar solvent, like H2O. The d- end of H2O is attracted to a cation (+). The d+ end of H2O is attracted to an anion (-). London Forces d+ 2e- 2e- d- d- Caused by the natural vibrations of the electrons in the bond, there is no permanent dipole moment. The dominant intermolecular force in nonpolar substances. d+ Hydrogen Bonding A specific case of Dipole – Dipole Forces. Occurs when H is involved in a strongly polar bond with O, N or F. The H nucleus (just a proton) is attracted to the lone pairs of these highly electronegative atoms. The result is a network of strong intermolecular interactions between H atoms and available lone pairs. Occurs extensively in H2O, NH3 and HF. The primary reason H2O doesn’t behave as almost all other substances in the known universe. H2O expands when it freezes everything else contracts Hydrogen Bonding Intermolecular Take-home Message The stronger the intermolecular interaction, the stronger molecules are held together. When molecules are held together more tightly, we see the evidence when we examine physical properties of the substance melting and boiling points increase Practice Questions For each of the following molecules, state which intermolecular force will be dominant. Then state which (out of each pair) should have the higher melting point. Molecule 1 Dominant Force Melting Point Molecule 2 Dominant Force Melting Point H2O H-Bonding 0 ºC H2S Dipole -82 ºC HCl Dipole -114.2 ºC H2 London -259 ºC NH3 H-bonding -78 ºC H2O H-bonding O ºC Br2 London -7 ºC I2 London 114 ºC Metals Metals, they’re an interesting bunch . . . They play by their own set of rules, so to describe them we need to talk about METALLIC bonding. First of all what do we know about the properties of metals? they’re malleable and ductile they’re electron rich (they give up their electrons to make cations) they conduct electricity the free flow of electrons yields many colorful solutions they’re shiny they conduct heat Metallic Bonding The “Sea of Electrons” Model Due to low electronegativities, low effective nuclear charges and large diffuse orbitals, electrons can flow freely from one atom to the next. Metallic Bonding The “Sea of Electrons” Model Electrons carry electrical current, if electrons can flow freely throughout the metal the metal will conduct electricity. Alloys A mix of 2 or more metals. 2 types: Substitutional and Interstitial Zn Cu Zn Fe Fe C Cu Zn Cu Fe Fe C Fe Fe Brass (Zn and Cu in various proportions) Steel (Fe and C in various proportions) Typically Zn is put in place of a Cu atom. C fills in the “holes” between the Fe atoms.