Polarity
advertisement

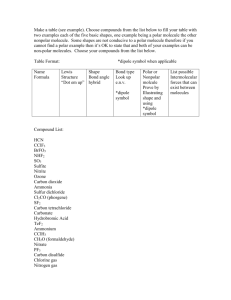
Honors Chemistry Polarity Worksheet Name: Date: To determine if a molecule is polar or not, there are several items that need to be taken into account. 1. The shape (molecular geometry) of the molecule. 2. The relative electronegativities of the elements. 3. The existence of any individual bond dipoles. 4. The existence of an overall, net dipole for the molecule, if it exists. There are several shapes that are always polar in nature, regardless of the electronectivities of the elements in the molecule. 1.Determine if the following molecules are polar or nonpolar. Do this by drawing an appropriate Lewis structure, labeling individual bond dipoles, and determining if there is an overall net dipole for the molecule. a. CI4 b.phosphorous triiodide c. carbon disulfide d. PF5 e. sulfur hexafluoride f. C2H6 g. SO2 h. HCN i. OF2 i. BF3 j. ozone k. xenon tetrafluoride