Polyatomic ions
advertisement

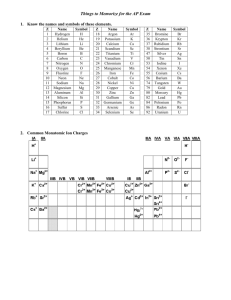
Polyatomic Ions + Na OH - Polyatomic ions A group of atoms covalently bonded together (molecule) with an overall charge (in other words, has gained or lost electrons so as a whole it is an ion) Ammonium NH4+ + Hydroxide OH- - Polyatomic ions Act as a unit Endings typically –ate or –ite Parenthesis used in formula if more than one polyatomic ion in a compound Counting Atoms/Molecules Please identify how many atoms/molecules in the following: Ca(NO3)2 ____ Ca _____ NO3 (NH4)3PO4 ____ NH4 _____ PO4 Na2CO3 ____ Na _____ CO3 Differentiated Activity Group 1: Bohr models Group 2: Finish powerpoint Example Find BIP and formula for sodium phosphate Sodium phosphate Na+ & PO43- B IP = 3 Na+ & 1 PO43Formula = Na3(PO4)1 = Na3PO4 Find the BIP and formula 1.) Barium phosphite 1.)BIP = 3Ba2+ & 2PO33Formula = Ba3(PO3)2 2.) Calcium nitrate 2.) BIP = 1Ca2+ & 2NO3Formula = Ca(NO3)2 3.) Rubidium chlorate 3.) B IP = 1Rb+ & 1ClO3Formula = RbClO3 Naming - Example What is the name of the following compound: Al(NO3)3 Aluminum nitrate Name NaOH NH4CN Ba(MnO4)2 Sodium hydroxide Ammonium cyanide Barium permanganate Reflection Write some simple things that will help you to remember how to write names and formulas for compounds with polyatomic ions Bond with a classmate OH- Mg2+ OH1 BIP: _______ Mg2+ & 2 ________ OH- Formula: Mg(OH)2