Things to Memorize for the AP Exam Know the names and symbols
advertisement

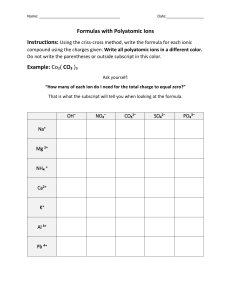
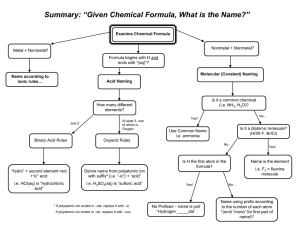
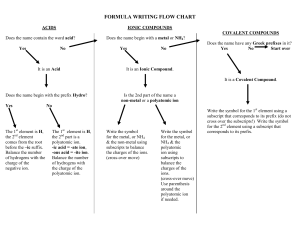
Things to Memorize for the AP Exam 1. Know the names and symbols of these elements. Z 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Name Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Symbol H He Li Be B C N O F Ne Na Mg Al Si P S Cl 2. Common Monatomic Ion Charges Z 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Name Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Symbol Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Z 35 36 37 38 47 50 53 54 55 56 74 79 80 82 84 86 92 Name Bromine Krypton Rubidium Strontium Silver Tin Iodine Xenon Cesium Barium Tungsten Gold Mercury Lead Polonium Radon Uranium Symbol Br Kr Rb Sr Ag Sn I Xe Cs Ba W Au Hg Pb Po Rn U Things to Memorize for the AP Exam 3. Polyatomic ion formulas and names. Polyatomic ions with odd group elements (e.g. N, Cl) have odd charge (usually -1) and polyatomic ions with even group elements (e.g. S, C) have even charge (usually -2). Adding H to a polyatomic ion increases charge by +1 (e.g. SO42-, HSO4-) 4. Selected solubility rules. Soluble salts have these ions: Group 1, NH4+, NO3-, HCO3-, ClO3-, C2H3O2- Exception none Insoluble salts have these ions: CO32-, PO43-, CrO42-, S2OH- Exception Group 1 & NH4+ Group 1 & Ba2+ Things to Memorize for the AP Exam 5. Activity series. Activity Series Most easily oxidized will displace H from water will displace H from acids will not displace H Most easily reduced