Ternary Ionic Compounds: Naming & Formulas
advertisement
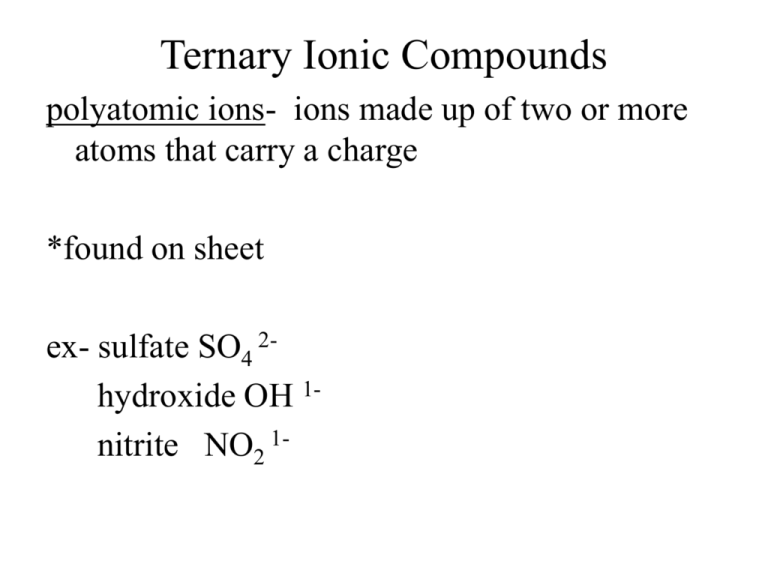
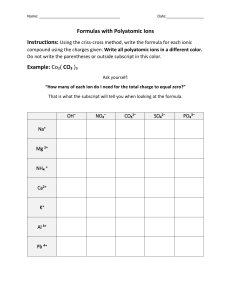
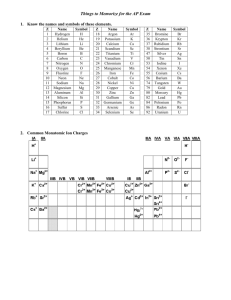
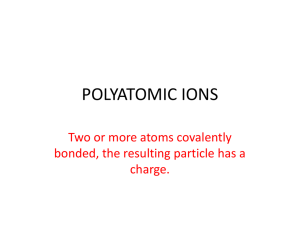
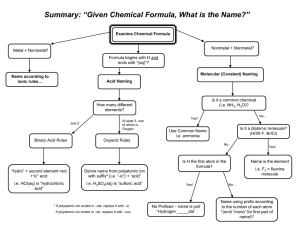
Ternary Ionic Compounds polyatomic ions- ions made up of two or more atoms that carry a charge *found on sheet ex- sulfate SO4 2hydroxide OH 1nitrite NO2 1- -most polyatomic ions are anions -only positive on sheet is: ammonium NH41+ Ternary Ionic Compounds -compounds that contain ions of usually three different elements with a polyatomic ion Rules for naming ternary ionic compounds 1) write the formula (symbol and charge) for each ion ex- calcium + nitrate Ca2+ NO312) criss cross charges Ca1NO32 3) you may need parentheses around polyatomic ion if a subscript greater than 1 goes there Ca1(NO3)2 4) lowest whole number ratios Ca(NO3)2 5) name cation then polyatomic anion calcium nitrate Examples: 1) potassium + sulfate K1+ SO42- K2SO4 potassium sulfate 2) tin (IV) + sulfite Sn4+ SO32Sn2(SO3)4 Sn(SO3)2 tin (IV) sulfite