Polyatomic Ions and Ionic Bonding
advertisement

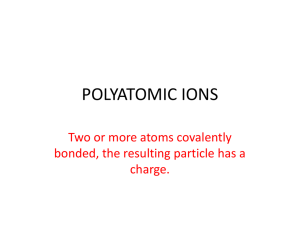
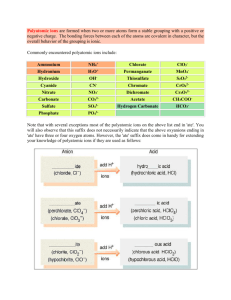
Polyatomic Ions and Ionic Bonding What we know…………… Ionic Bonds form between a metal and a non-metal In an ionic bond, electrons are transferred from the metal (cation) to the non-metal (anion) Polyatomic Ions An ion made up of two or more elements Elements stay together and act as one unit in chemical reactions Together, the elements still carry charge Ex) hydroxide = OH- Common Polyatomic Ions Writing Formulas with Polyatomics Follow the same procedure as we learned last class, with one small exception. If more than one Polyatomic Ion is needed to balance the charges, you must use parentheses around the ion before writing subscript Ex: Magnesium Hydroxide (Mg+2, OH-1) Incorrect Formula: MgOH2 Correct Formula: Mg(OH)2 Formula Examples Sodium Phosphate: Na+ + PO43- = Na3PO4 Magnesium Phosphate: Mg2+ + PO43- = Mg3(PO4)2 Naming with Polyatomics Good News! You just use the name of the polyatomic ion! Ex: CaCO3 Ca = calcium, CO3 = carbonate Name = calcium carbonate Writing Polyatomics in Formulas Write the formulas for the following compounds: Strontium Nitrite Calcium Hydroxide Iron (III) Carbonate Copper (II) Sulfate Ammonium Sulfide Ammonium Nitrate Naming with Polyatomic Ions Write the names of the following polyatomic ions: Mg(OH)2 Na2SO4 Al2(SO4)3 Pb3(PO4)4 NH4NO3 Mixed Practice - Naming Write the names for the following compounds AgCl3 Fr3N Fe3(PO4)2 CuCO3 NaCl Sn(CO3)2 NH4OH Mixed Practice - Formulas Write the formulas for the following compounds Magnesium Peroxide Tin (IV) Chromate Ammonium Phosphate Copper (II) Permanganate Mercury (I) Chloride Ammonium Oxide Strontium Phosphide Aluminum Iodide