DOUBLE REPLACEMENT REACTIONS
advertisement
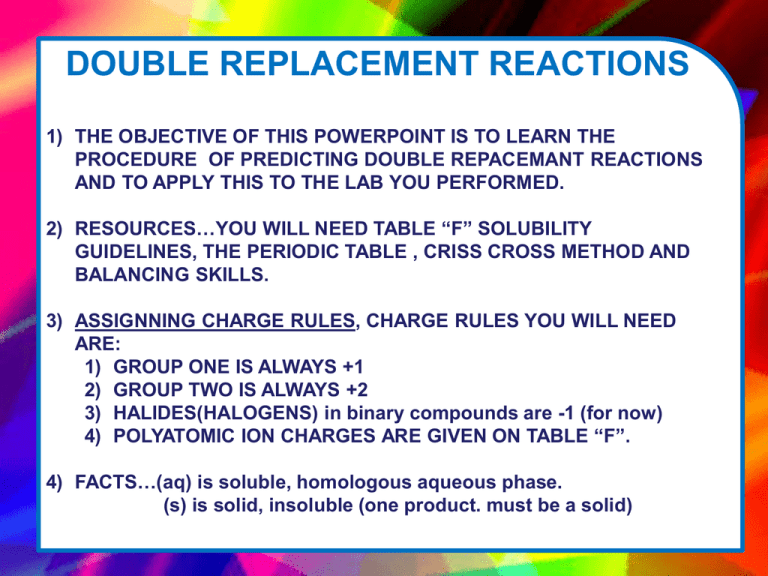
DOUBLE REPLACEMENT REACTIONS 1) THE OBJECTIVE OF THIS POWERPOINT IS TO LEARN THE PROCEDURE OF PREDICTING DOUBLE REPACEMANT REACTIONS AND TO APPLY THIS TO THE LAB YOU PERFORMED. 2) RESOURCES…YOU WILL NEED TABLE “F” SOLUBILITY GUIDELINES, THE PERIODIC TABLE , CRISS CROSS METHOD AND BALANCING SKILLS. 3) ASSIGNNING CHARGE RULES, CHARGE RULES YOU WILL NEED ARE: 1) GROUP ONE IS ALWAYS +1 2) GROUP TWO IS ALWAYS +2 3) HALIDES(HALOGENS) in binary compounds are -1 (for now) 4) POLYATOMIC ION CHARGES ARE GIVEN ON TABLE “F”. 4) FACTS…(aq) is soluble, homologous aqueous phase. (s) is solid, insoluble (one product. must be a solid) EXAMPLE REACTIONS 1) FIRST…EXCHANGE THE METALS TO GET PRODUCTS Na3 (PO4) (aq) + Ba(NO3)2 (aq) Na3 (PO4) + Ba(NO3)2 Ba(PO4) +Na(NO3) 2) USE CRISS CROSS TO DERIVE CORRECT SUBSCRIPS OF PRODUCTS. REMEMBER, SUBSCRIPTS OF ELEMENTS ARE NOT INHERETED ACROSS THE ARROW. NEVER CHANGE A POLYATOMIC ION FORMULA, HOWEVER THE SUBSCRIPT OUTSIDE OF ITS PARENTHESIS IS NOT INHERITED. • EXAMPLE REACTIONS 2) USE THE CRISS CROSS METHOD TO DETERMINE THE CORRECT SUBSCRIPTS OF THE PRODUCTS. • Na3 (PO4) + Ba(NO3)2 Ba(PO4) +Na(NO3) Ba(PO4) Ba is in group 2 and is +2 each Ba+2 Phosphate is -3 each (table E) (PO4) -3 Na(NO3) Na is in group 1 and is +1 each Na+1 NO3 is -1 from table E (NO3) -1 Na1(NO3)1 Ba3 (PO4)2 Na(NO3) Barium Phosphate Sodium Nitrate • EXAMPLE REACTIONS 3) THIRD…WITH THE CORRECT PRODUCT SUBSCRIPTS IN PLACE, USE TABLE “F” TO DETERMINE IF ONE OF THE PRODUCT SALTS IS INSOLUBLE. • Na3 (PO4)(aq) + Ba(NO3)2(aq) Ba3(PO4)2 (s) +Na(NO3)(aq) From table “F” all Phosphates are insoluble, Barium is not an exception. All nitrates are soluble, no exceptions 4) Now its time to balance…your favorite! Use MINOH… M – balance metals first I – balance polyatomic ions second N- Balance non metals O-balance oxygen H-balance hydrogen EXAMPLE REACTIONS 4)BALANCE( MINOH pt 1)---BALANCE METALS • Na3 (PO4)(aq) + 3 Ba(NO3)2 (aq) Ba3(PO4)2 (s) + 3 Na(NO3)(aq) 4) BALANCE(MINOH pt 2)---BALANCE IONS (POLYATOMIC IONS)—(THERE ARE NO NONMETALS TO BALANCE SO WE GO DIRECTLY TO IONS.) • 2 Na3 (PO4)(aq) + 3 Ba(NO3)2 (aq) Ba3(PO4)2 (s) + 3 Na(NO3)(aq) THE COEFFICIENT 2 BALANCES THE 2 PHOSPHATES ON THE RIGHT 2 Na3 (PO4)(aq) + 3 Ba(NO3)2 (aq) Ba3(PO4)2 (s) + 63 Na(NO3)(aq) THE COEFFICIENT 6 BALANCES THE 6 NITRATES ON THE LEFT AND THE 6 SODIUMS ON THE LEFT. BALANCED NOW! NOW…DETERMINE THE IONIC REACTION… DISSOCIATE THE (aq) SALTS. 2 Na3 (PO4)(aq) + 3 Ba(NO3)2 (aq) Ba3(PO4)2 (s) + 6 Na(NO3)(aq) 6 Na+ + 2 (PO4)3- + 3 Ba+2 + 6 (NO3)- Ba3(PO4)2 (s) + 6 Na+ + 6 (NO3)- FINNALY….cancel spectator ions (ones that did not change phase) to derive NET IONIC REACTION 6 Na+(aq)+ 2 (PO4)3-(aq) + 3 Ba+2 (aq) + 6 (NO3)- (aq) Ba3(PO4)2 (s) + 6 Na+ (aq) + 6 (NO3)- (aq) THE NET IONIC EQUATION IS ………………….. 2 (PO4)3-(aq) + 3 Ba+2 (aq) Ba3(PO4)2 (s)