Polyatomic Ions - Trinity Valley School
advertisement

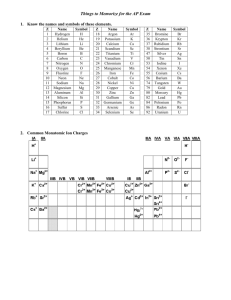
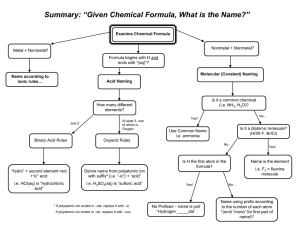
Polyatomic Ions Many of the ionic compounds we deal with in chemistry are called binary compounds. They are called this because they consist of two different elements. In the case of an ionic compound, one element is a monatomic cation, and the other, a monatomic anion. NaCl, or sodium chloride, is a classic example. In class we have learned how to write formulas and names for these types of compounds. Ionic compounds can also be formed from polyatomic ions as well. A polyatomic ion is an ion that is formed from a group of two or more nonmetal elements. The entire group carries a specific charge and acts as one unit. A classic example of a compound that contains a polyatomic ion is blackboard chalk, or calcium carbonate. The formula for chalk is CaCO3, and consists of the monatomic cation calcium, Ca2+, and the polyatomic anion CO32-, which is given the name carbonate. The entire CO3 carries the – 2 charge, and acts as a group just as Cl- does in sodium chloride. Unfortunately, the only way to learn the polyatomic ions is by memorization and practice. We will divide this exercise up into two parts. The first set of ions you must know for Test III.. You are responsible for the second set by the time of our next quiz in Nov after test III. This set can be found on page 127 of your text. Polyatomic ions you need you need to know – Round I Name Formula ammonium NH4+ acetate C2H3O2cyanide CNhydroxide OHoxalate C2O42sulfite SO32sulfate SO42nitrite NO2nitrate NO3carbonate CO32perchlorate ClO4chlorate ClO3chlorite ClO2hypochlorite ClOphosphate PO43Phosphite PO33=