BONDING SKILL TWO – POLYATOMIC IONS.
advertisement
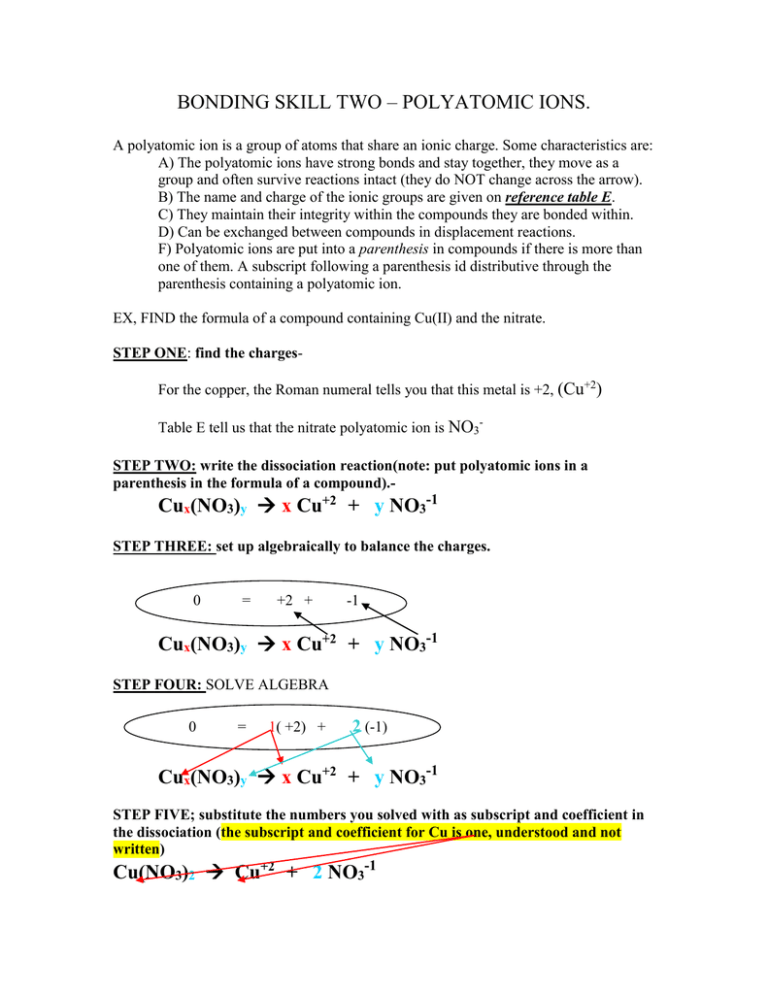
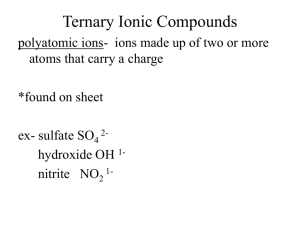
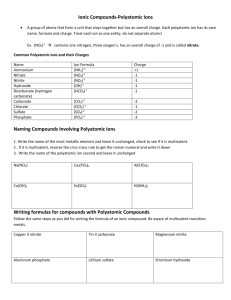
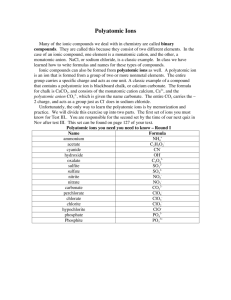
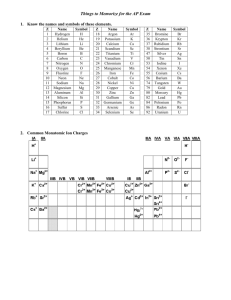
BONDING SKILL TWO – POLYATOMIC IONS. A polyatomic ion is a group of atoms that share an ionic charge. Some characteristics are: A) The polyatomic ions have strong bonds and stay together, they move as a group and often survive reactions intact (they do NOT change across the arrow). B) The name and charge of the ionic groups are given on reference table E. C) They maintain their integrity within the compounds they are bonded within. D) Can be exchanged between compounds in displacement reactions. F) Polyatomic ions are put into a parenthesis in compounds if there is more than one of them. A subscript following a parenthesis id distributive through the parenthesis containing a polyatomic ion. EX, FIND the formula of a compound containing Cu(II) and the nitrate. STEP ONE: find the chargesFor the copper, the Roman numeral tells you that this metal is +2, (Cu+2) Table E tell us that the nitrate polyatomic ion is NO3STEP TWO: write the dissociation reaction(note: put polyatomic ions in a parenthesis in the formula of a compound).-1 +2 Cux(NO3)y x Cu + y NO3 STEP THREE: set up algebraically to balance the charges. 0 = +2 + -1 Cux(NO3)y x Cu+2 + y NO3-1 STEP FOUR: SOLVE ALGEBRA 0 = 1( +2) + 2 (-1) Cux(NO3)y x Cu+2 + y NO3-1 STEP FIVE; substitute the numbers you solved with as subscript and coefficient in the dissociation (the subscript and coefficient for Cu is one, understood and not written) -1 +2 Cu(NO3)2 Cu + 2 NO3