5.9 Polyatomic Ions
advertisement

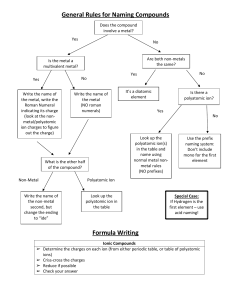
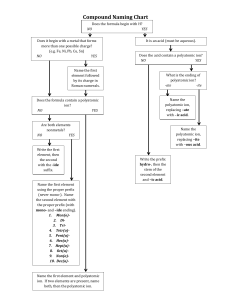
Ionic Compounds-Polyatomic Ions A group of atoms that form a unit that stays together but has an overall charge. Each polyatomic ion has its own name, formula and charge. Treat each ion as one entity, do not separate atoms! Ex. (NO3)-1 contains one nitrogen, three oxygen's, has an overall charge of -1 and is called nitrate. Common Polyatomic Ions and their Charges Name Ammonium Nitrate Nitrite Hydroxide Bicarbonate (hydrogen carbonate) Carbonate Chlorate Sulfate Phosphate Ion Formula (NH4)+1 (NO3)-1 (NO2)-1 (OH)-1 (HCO3)-1 Charge +1 -1 -1 -1 -1 (CO3)-2 (ClO3)-1 (SO4)-2 (PO4)-3 -2 -1 -2 -3 Naming Compounds Involving Polyatomic Ions 1. Write the name of the most metallic element and leave it unchanged, check to see if it is multivalent. 2. If it is multivalent, reverse the criss-cross rule to get the roman numeral and write it down 3. Write the name of the polyatomic ion second and leave it unchanged Na(NO3) Ca3(PO4)2 Al(ClO3)3 Cu(OH)2 Sn(SO4) Ni(NH4)3 Writing formulas for compounds with Polyatomic Compounds Follow the same steps as you did for writing the formula of an ionic compound. Be aware of multivalent transition metals. Copper II nitrate Tin II carbonate Magnesium nitrite Aluminum phosphate Lithium sulfate Strontium hydroxide