REACTIONS OF ALCOHOLS
advertisement

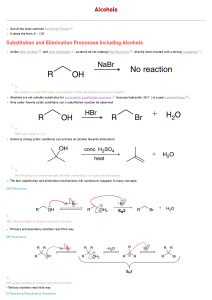
REACTIONS OF ALCOHOLS 1. Combustion (Extreme Oxidation) a l c o h o l+ o x y g e n c a r b o n d i o x i d e + w a t e r 2 C H C H O H + 6 O 4 C O + 6 H O 3 2 2 2 2 2. Elimination (Dehydration) ° H S O / 1 0 0 C 2 4 a l c o h o l a l k e n e + w a t e r ° H S O / 1 0 0 C C H C H C H O H C H C H = C H + H O 3 2 2 3 2 2 2 4 3. Condensation ° H S O / 1 4 0 C 2 4 e x c e s s a l c o h o l ° e t h e r+ w a t e r H S O / 1 4 0 C 2 C H C H O H C H C H O C H C H + H O 3 2 3 2 2 3 2 2 4 4. Substitution L u c a s R e a g e n t Z n C l 2 a l c o h o l+ h y d r o g e n h a l i d e a l k y lh a l i d e + w a t e r Z n C l C H C H O H + H C l C H C H C l+ H O 3 2 3 2 2 2 • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol turns cloudy after 5 minutes • Primary Alcohol takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate chromium 3+ (orange) (green) permanganate manganese (IV) oxide (purple) (brown) T e r t i a r y A l c o h o l n o to x i d i z e d u n d e rn o r m a lc o n d i t i o n s C H 3 K M n O 4 H C C O H 3 K C r O 2 2 7 N O R E A C T I O N C H 3 t e r t b u t y la l c o h o l S e c o n d a r y A lc o h o l k e to n e+ h y d r o g e n io n s H O K M n O 4 H C 3 C C H 3 O H 2 p r o p a n o l P r i m a r y A l c o h o l + +2 H C K C r O 2 2 7 H C 3 C H 3 p r o p a n o n e a l d e h y d e + w a t e r c a r b o x y l i c a c i d + h y d r o g e n i o n s OH OH H K M n O H 4 K M n O 4 + C H C H C H O H CCC CCC H + 2 H 3 2 2 H + H O K C r O 2 K 2 2 7 C r O 2 2 7H O H H H H H 1 p r o p a n o l p r o p a n o i c a c i d p r o p a n a l 6. Acid-Base Reactions • Like water, alcohols can act as an acid or base, depending on what it is reacting with. • When they react as an acid, the alkyl oxide ion (R-CH2O-) is formed. e t h a n o l+ s o d i u m e t h o x i d e i o n + s o d i u m i o n + h y d r o g e n 2 C H C H O H + 2 N a 3 2 + 2 C H C H O + 2 N a + H 3 2 2 • When they react as a base, the alkyl oxonium ion (R-CH2OH2+) is formed e t h a n o l+ s u l f u r i c a c i d C H C H O H + H S O 3 2 2 4 e t h y l o x o n i u m i o n + b i s u l f a t e i o n + C H C H O H H S O 3 2 2+ 4 Preparation of Alcohols 1. Hydration of an Alkene a lk e n e+w a te r ° H S O 0 0 C 2 4/1 a lc o h o l ° H S O 0 0 C 2 4/1 H CC H C H O 2 3 +H 2 HH H H C C C H HH HO 2. Oxidation of an Alkene • This reaction uses an oxidizing agent like KMnO4 or K2Cr2O7 to produce a “diol”. a lk e n e+w a te r H CC H C H O 2 3 +H 2 K M n O 4 K C r2O 2 7 K M n O 4 K C r2O 2 7 " d io l" HH H HC C C H HH O HO Reactions of Ethers 1. 2. 3. Ethers do not react with oxidizing or reducing agents. Combustion ether + oxygen carbon dioxide + water CH3-O-CH3 + 3 O2 2 CO2 + 3 H2O Reaction with Concentrated Binary Acids e th e r+ 2 b in a r y a c id C H O C H C H 2 H C l 3 2 3+ 2 a lk y lh a lid e s+ w a te r H C C l+ C lC H C H H O 3 2 3+ 2 4. Reaction with Atmospheric Oxygen This is a slow reaction in which highly unstable peroxides are formed ether+oxygen C H C H H 3O 2C 3+O 2 peroxide H O O C H H 3C 2C 3