Oxidation of Alcohols
advertisement

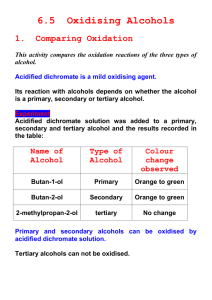
What is combustion? Write a definition Write an equation of combustion for the following alcohols: • Ethanol • Methanol • Propanol • Combustion – The process of burning by reacting a fuel with oxygen • C2H5OH (l) + 3O2 (g)2CO2 (g) + 3H2O (l) • CH3OH (l) + 1½O2 (g)CO2 (g) + 2H2O (l) • C3H7OH (l) + 4½O2 (g)3CO2 (g) + 4H2O (l) Oxidation of Alcohols Oxidation of Alcohols • Primary and secondary alcohols can be oxidised using an oxidising agent. • Suitable oxidising agent is a solution containing acidified dichromate ions H+ /Cr2O72• During the reaction, the acidified potassium dichromate changes colour from orange to green. • Alcohols fall into different classes depending on how the -OH group is positioned on the chain of carbon atoms • In a primary (1°) alcohol, the carbon which carries the -OH group is only attached to one alkyl group. • In a secondary (2°) alcohol, the carbon with the OH group attached is joined directly to two alkyl groups Primary Alcohols • On gentle heating with potassium dichromate a primary alcohol can be oxidised to produce an aldehyde. On stronger heating in excess acidified dichromate alcohol will pass through aldehyde stage to form carboxylic acid. • Reflux – the continual boiling and condensing of a reaction mixture to ensure that the reaction takes place without the contents of the flask boiling dry. Secondary and Tertiary Alcohols • Oxidised by dichromate ions to produce ketones. Ketones can’t be oxidised any further • Tertiary alcohols are resistant to oxidation so don’t react.