Alcohols and Ethers
advertisement

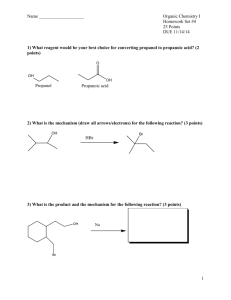
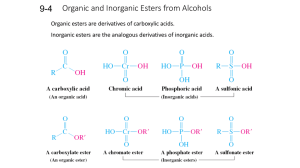
Alcohols and Ethers Read pp. 204-208 Alcohols Organic compound that has a hydroxyl group (OH) attached to an alkyl group (an alkane minus one hydrogen). General formula: R-OH (where R is alkyl group) Structural Formulas for Alcohols 1. Draw carbon backbone 2. Add –OH group in right spot 3. Fill in hydrogen bonds Draw: a) 1-butanol b) 2-pentanol Naming Alcohols 3-hexanol 3-heptanol Properties of Alcohols • The longer the alcohol molecule, the higher the boiling point • More soluble (can dissolve) in water than alkanes (due to presence of –OH group) Ethers Organic compound that has a single oxygen atom bonded to two separate hydrocarbon chains. General Formula: R-O-R (where R is alkyl group). Naming Ethers Ethers are recognized by the presence of “oxy” in the IUPAC name. You add “oxy” right after the prefix of the SMALLER hydrocarbon chain, followed by the LARGER alkane name. HH HHH H HHHH H-C-C-O-C-C-C-H H-C-O-C-C-C-C-H HH HHH H HHHH ethoxypropane methoxybutane Structural Formulas of Ethers Draw the following ethers: methoxypentane H HHHHH H-C-O-C-C-C-C-C-H H HHHHH butoxyhexane HHHH HHHHHH H-C-C-C-C-O-C-C-C-C-C-C-H HHHH HHHHHH Properties of Ethers • More polar than hydrocarbons (due to presence of oxygen atom) • Boiling point is slightly higher than hydrocarbons, but lower than alcohols