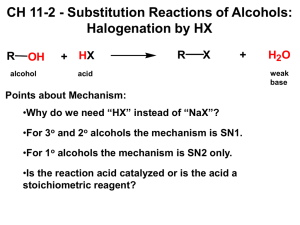C341 Final Exam Study Guide
advertisement

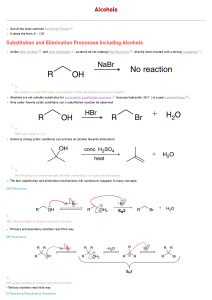
C341 Final Exam Study Guide Summer 2009 General information: 8:00 AM- 11:00AM on Friday, August 14 in Rawles 100 Format: 200 points, cumulative, similar format to other exams Approximate point distributions: ~75 pts mechanism Draw the mechanism (substitution, elimination, addition) Answer questions based on mechanisms ~70 pts reagents, reactions Predict the major product with stereochemistry Provide the correct reagents ~65 pt miscellaneous concepts from list below Tips for studying: Work from the end of the course to the beginning (study backwards) Print blank discussion problem sets and write full answer before checking in the Smith text Retake old exams and quizzes, then check your answers Do practice chapter problems listed at the end of the syllabus A list of major topics: Formal charge, resonance structures, hybridization, skeletal structures, acid/base equilibria, pKa’s, trends in acidity/basicity, functional groups, intermolecular forces, alkane nomenclature, conformational analysis, Newman projections, causes of strain, cyclohexane ring structures, chiral, achiral, R/S nomenclature, enantiomers, diastereomers, optically active, meso compounds, arrow mechanisms, transition states, rate limiting steps, activation barrier, exothermic/endothermic reactions, nucleophile, leaving group, substitution reactions, SN1 mechanism, SN2 mechanism, alkene classifications, alkene stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, E/Z nomenclature, addition reactions, Markovnicov’s rule, anti-marcovnicov product, regiochemistry of additions, stereochemistry of additions, reactions of alkynes, acidity of terminal alkynes, multistep synthesis, redox reactions, reagents for redox reactions, allylic brominations, conjugation, 1,4 additions, Diels-Alder reaction, Kinectic vs thermodynamic product