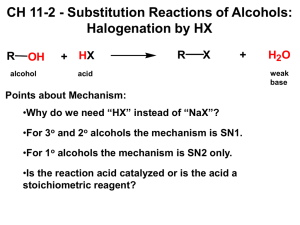Uploaded by
Dafpht The Mouthbreather
Alcohols: Substitution & Elimination Reactions Explained
advertisement

Alcohols One of the most common Functional Groups It takes the form 6 R − OH Substitution and Elimination Processes Including Alcohols Unlike Alkyl Halides 24 and Alkyl Sulfonates 4 , alcohols do not undergo SN2 Reactions 10 directly when treated with a strong nucleophile 7 . 1 No reaction is observed when a weak base strong nucleophile is used as reagent Alcohols are not suitable substrates for nucleophilic substitution reactions 10 because hydroxide Only under heavily acidic conditions can a substitution reaction be observed: 1 HBr's pKa value is -8.7 Similarly strong acidic conditions can activate an alcohol towards elimination: 1 The OH group can be protonated, thereby converting it to a good leaving group The four substitution and elimination mechanisms will continue to reappear in many concepts SN2 Reactions: 1 HBr allows alcohol to convert into alkyl bromide Primary and secondary alcohols react this way SN1 Reactions: 1 HBr allows alcohol to convert into alkyl bromide - Tertiary alcohols react this way E1 Reactions/Dehydration Reactions: (H O − ) is a poor Leaving Group 18 . 1 H 2 SO 4 plus heat produces H3 O + , allowing the alcohol to be protonated by converting it into a good leaving group This process involved the formation of an alkene 19 via the removal of water, making it therefore a dehydration reaction Dehydration of an alcohol under acidic conditions occurs via a three-step mechanism 1. Proton Transfer to form carbocation is the strongest acid that can be used in a solvent due to the Levelling Effect 1 so is just going to be represented by the reaction 2. Loss of a leaving group 3. Proton Transfer to form alkene H3 O + H 2 SO 4 E2 Reactions/Dehydration Reactions: Some alcohols can yield more than one possible alkene from dehydration 1 The more substituted alkene is generally favored as expected Dehydration is also observed to occur with secondary or primary substrates 1 In this case, dehydration cannot occur via an E1 process because the formation of the require 1 This reaction is more likely to go through an E2 process after the protonation of OH 1 Under these strongly acidic conditions, OH group is protonated followed by an process to give the alkene It is not possible to protonate the alcohol and then react the resulting with a strong base to give an reaction E2 ROH Strong base acid An alcohol can only be protonated under strongly acidic conditions + 2 E2 H3 O + in Examples: Predict the major product for each of the following reactions: 1 Answer: a. 1 b. 1 1 Answer: a. 1 b. 1 c. 1 d. 1