Practice exam questions on Hydrocarbons
advertisement

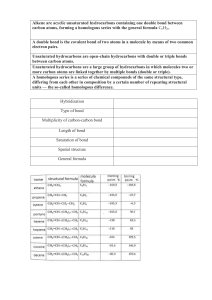
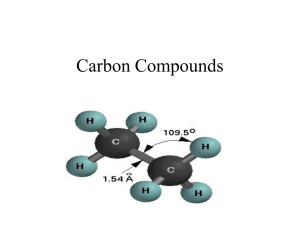
Practice exam questions on Hydrocarbons: 1. A. B. C. D. Which of the following semi-structural formulae would be that for a carboxylic acid? CH3CH2CH2OH CH3CH2CHO CH3CH2COOH CH3COOCH3 2. Which of the following statements best describes the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? A. Unsaturated hydrocarbons only have single carbon-carbon bonds in their structures whereas saturated hydrocarbons can have one or more double or triple carbon-carbon bonds. B. Saturated hydrocarbons only have single carbon-carbon bonds in their structures whereas unsaturated hydrocarbons can have one or more double or triple carboncarbon bonds. C. Saturated hydrocarbons will completely dissolve in water whereas unsaturated ones will not. D. Saturated hydrocarbons undergo more reactions than unsaturated ones. 3. A. B. C. D. The molecule making up the gas ethene is best described as a planar molecule with a double covalent bond between the carbon atoms three dimensional network lattice with a single bond between the carbon atoms planar molecule with a single covalent bond between the carbon atoms three dimensional network lattice with a double bond between the carbon atoms. Question 1 (2 marks) Write appropriate balanced chemical equations for each of the following. Include states for each reactant and product. (a) Pentane gas burns in a good supply of air to form carbon dioxide and water vapour. ______________________________________________________________________ Question 2 (4+4+1=9marks, 8 minutes) (a) Draw structures for the two isomers of the hydrocarbon C4H10, and give their systematic names. (b) Draw structural formulae for the following molecules, clearly showing the shape. (i) ethane (ii) pentanoic acid (iii) methanol (iv) ethyne (c) The masses of ethane and methanol molecules are fairly similar, yet the boiling points of the two materials are significantly different with ethane boiling at -89oC while methanol boils at 65oC. Why is the boiling point of methanol higher than that for ethane? ______________________________________________________________________ ______________________________________________________________________