Unit 4: Chemical Reactions and Solutions
advertisement

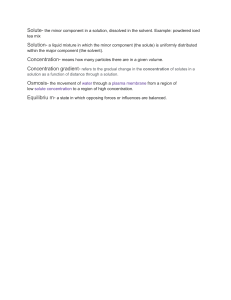
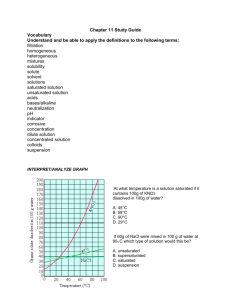
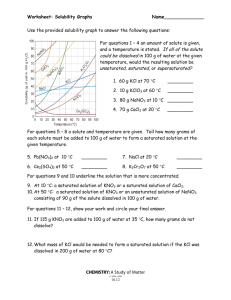
Unit 6: Chemical Reactions and Solutions Study Guide Complete this study guide ON A SEPARATE SHEET OF PAPER and submit it on the day of the test for up to 10 EC points on the test. In addition to the information covered by this study guide, test questions will also be drawn from classwork and classnotes. Reviewing all of this information is STRONGLY recommended. Define: Catalyst Coefficient Inhibitor Saturated solution Solubility Solute solvent Subscript Unsaturated solution Questions: 1. What is the general form of a chemical reaction equation? 2. Where is the chemical energy in a compound found? 3. Draw a reaction energy diagram for an endothermic and exothermic reaction. Label the position of the reactants, products and energy barrier. 4. For the reactions in #3, write out a general chemical equation that includes energy. 5. How does concentration, temperature and particle size affect the rate of a reaction? 6. How would a catalyst change the reaction energy diagrams you drew in question #3? 7. What are the four types of reactions that we discussed in class and how can each type be identified? 8. How is a solution prepared? 9. How can a saturated and an unsaturated solution be distinguished? 10. How does temperature affect the dissolution rate of solid, liquid and gaseous solutes? 11. What happens to the freezing and boiling point of a solvent when a solute is dissolved in it? 12. What is true about temperature during a phase change? 13. Sketch a heating curve for a substance that melts at -20C and boils at 50C. Label the phases that are present at each interval. 14. How are volume and shape described for solids, liquids and gases in terms of the particles in them?