AP Chemistry Thermochemistry Reading Guide
advertisement

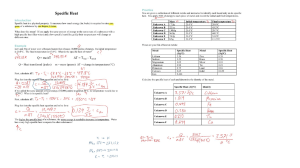
AP Chemistry – Chapter 5 Reading Guide: Thermochemistry 1. List and give examples of the different forms of energy. 2. What are the 2 units of energy that are used in Chemistry? How can one convert between these? 3. Define the first law of thermodynamics both verbally and by means of an equation. 4. Distinguish between endothermic and exothermic processes. What does the algebraic sign on the enthalpy change tell you? 5. Describe how the change in internal energy of a system is related to the exchanges of heat and work between the system and surroundings. 6. Define the word state function and describe its importance in thermochemistry. 7. Define enthalpy, and relate the enthalpy change in a process occurring at constant pressure to the heat added or lost by the system during the process. 8. Sketch a potential energy diagram, one for an endothermic process and one for an exothermic process. Label ∆H. (You will need to google this as it’s not in your book!) 9. Differentiate between specific heat and heat capacity. 10. Explain calorimetry and what equation is used to perform these calculations. 11. Define what is meant by standard state. Identify the standard states of Oxygen, Carbon, and Hydrogen. 12. Define the term standard heat of formation, and identify the type of chemical reaction with which it is associated. 13. Explain how to calculate the enthalpy change in a reaction occurring at constant pressure, given a table with the standard enthalpies of formations of each reactant and product.