Interpreting Potential Energy Diagrams
advertisement

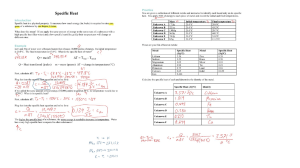
Interpreting Potential Energy Diagrams Practice Graph 1 1. What type of reaction (exothermic or endothermic) does the graph represent? 2. Calculate the following: i) ∆H = _________ ii) Ea = __________ 3. Would you predict this reaction to happen at room temperature? List 2 reasons for your answer. 4. Calculate the ∆H and Ea for the reverse reaction. Graph 2 1. What type of reaction (exothermic or endothermic) does the graph represent? 2. Calculate the following: i) ∆H = _________ ii) Ea = __________ 3. Would you predict this reaction to happen at room temperature? List 2 reasons for your answer. 4. Calculate the ∆H and Ea for the reverse reaction. Graph 3 Consider the following reaction: A C i) What does B represent in the reaction? ii) Is the overall reaction exothermic or endothermic? iii) On the graph, draw a line to represent the net enthalpy change (heat of reaction). iv) What does [X]F represent? v) Which step would you expect to be the rate-determining step? Why?