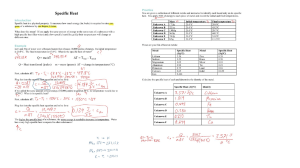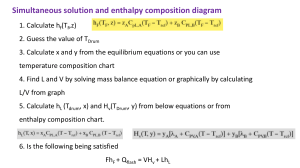
ENERGY BALANCE UNIT V ENERGY BALANCE • Heat capacity of solids, liquids, gases and solutions, Use of mean heat capacity in heat calculations, • Problems involving sensible heat and latent heats, Evaluation of enthalpy. • Standard heat of reaction, heat of formation, combustion, solution, mixing etc., • Calculation of standard heat of reaction, • Effect of pressure and temperature on heat of reaction. • Unsteady state energy balances. COURSE OUTCOMES Perform energy balance calculations for various processes in Chemical Engineering. Energy Balance Physical or Chemical changes in matter ~ accompanied by Enthalpy changes Energy Balance In chemical reaction ~ change in enthalpy always occurs Energy Balance Energy level diagrams of Exothermic and Endothermic Reaction Energy Balance . Energy Balance Energy Balance Work done increases the Potential Energy Energy Balance Potential Energy → Kinetic Energy Energy Balance Loss of work done → Thermal Energy Energy Balance Reaction → Chemical Energy Energy Balance Mechanical work done → Electrical Energy Energy Balance Mechanical work → Energy Energy Balance Energy → Mechanical work Energy Balance Unit of Work = Unit of Energy Energy Balance Energy Balance Aim of Thermochemistry Energy Balance Terminology in Thermochemistry Energy Balance First Law of Thermodynamics: “Energy can neither be created nor destroyed during a process although it can be converted from one form to another.” (or) “The total energy of an isolated system and its surrounding remains constant.”