PowerPoint - Thermochemistry, Heat Capacity, and
advertisement

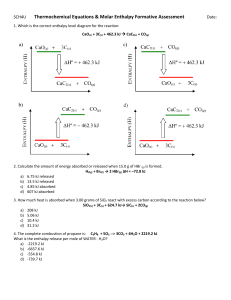
Specific heat capacity (a.k.a. Specific heat) • symbolized as c, units in J/gC • It’s the heat required to raise 1 gram of a substance by 1 C Heat capacity • calculated by c x m, units in J/C • It’s the heat required to raise the temperature of an object by 1 C. Molar heat capacity • Similar to specific heat capacity, but uses moles instead of grams, units in J/mol C. • It’s the heat required to raise the temperature of 1 mol of a substance by 1 C Answers 2. a) As calculated yesterday: 25.5 kJ b) Specific heat is 4.18 J/gC (pg. 151) c) Heat capacity: cm=4.18J/gCx200g=836 J/C d) Molar heat capacity is c x g/mol =4.18 J/gC x 18 g/mol = 75.3 J/molC 3. Simply using q=cmT, c= 4.18 J/gC, m=335 g, T=26.4-24.5= 1.9C q=cmT= 4.18 J/gC x 335 g x 1.9C = 2.7 kJ 4. We cannot determine the c for wax from this data because we did not heat the candle (no value for T). We would have to change the lab procedure if we wanted c for wax. 5. q=cmT= 2.0 J/gC x 5 g x 60 C = 600 J 6. q=cmT, c=q/mT= 10 J /(3.1 g)(17.9C) = 0.18 J/gC (gold is 0.129 J/gC - pg. 151; it is not pure) heat capacity = cm = 0.18 J/gC x 3.1 g = 0.558 J/ C 5.15: Something with a high c needs more energy to increase in temperature 5.16: Something with a low c experiences a greater rise in temp. given the same energy 5.18: The body is mostly water. Water (and the body) has a high c, thus a large change in energy will cause only a small shift in temp. Crossword 8. negative 9. positive 11. grams 12. bomb 14. law of conservation of energy 17. insulator 18. endothermic 19. oxygen 20. water 1. 2. 3. 4. 5. 6. 7. 10. 13. 15. 16. enthalpy change thermochemistry calorimeter heat of reaction exothermic out surroundings boundary enthalpy system coffeecup 9. Enthalpy (total energy) includes: chemical potential energy of chemicals gravitational potential energy kinetic energy (earth’s rotation, etc) heat energy We cannot measure enthalpy because we cannot measure absolute gravitational or kinetic energy 10.Any reaction that produces light, sound, kinetic energy (explosion), etc. 11. Thermometer identifies T of water. H is found using q=cmT and law of conservation of energy (technically, pressure must be constant for q to equal H) Boundary Endothermic Exothermic System A basic calorimeter - see handout • In our lab we tried to determine H via q from “calorimetry”. Here are some terms associated with calorimetry (and thermochemistry) Surroundings everything else System with can as boundary Endothermic = absorbing energy Exothermic = releasing energy Law of conservation of energy = release and absorption of energy must be equal For more lessons, visit www.chalkbored.com