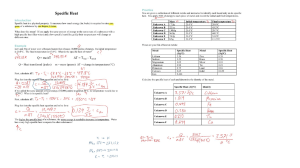
Year 11 Chemistry Worksheet Name: Data: Q1. There are two types of chemical reactions Q2. There are many different forms of energy such as Q3. The SI unit of energy is _______________________________ Q4. 1MJ = ______________KJ = ____________________J = 1.0 x 106 J Q5. What is law of conservation of energy? Q6. In Chemistry, the system is usually the _______________________________. Q7. The surroundings are usually regarded as _____________________________. Q8. Energy will be _______________ or __________________________ during a chemical reaction. Q9. The chemical reaction in which energy is ________________________ is called exothermic reaction. Q10. The chemical reaction in which energy is ________________________ is called endothermic reaction. Q11. What is an endothermic and an exothermic process? Q13. All reactions absorb some energy before they can proceed, even if energy is released overall. (True / False) Q14. The enthalpy of the reactant is represented by a symbol ________________________ The enthalpy of the product is represented by a symbol ________________________ Q15. Define enthalpy change. Q16. When Hp is _____________ than Hr, energy is released from the system into the surroundings so the reaction is _____________________. The system has lost energy so has ______ value. Q16. When Hp is _____________ than Hr, energy is absorbed from the surroundings into the system so the reaction is _____________________. The system has lost energy so has ______ value. Q17. If the coefficients of the thermochemical equation are doubled, the __________. will also be