3 heat content or enthalpy is a thermodynamic variables and... increase in enthalpy of a system accompanying a change in...
advertisement

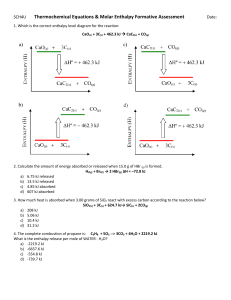
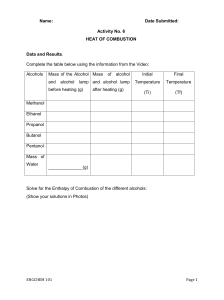
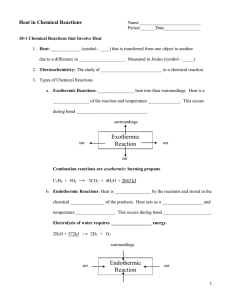
3 heat content or enthalpy is a thermodynamic variables and an extensive property . the increase in enthalpy of a system accompanying a change in the system at constant pressure equal to the heat absorbed by the system. =(UB+PVB)-(UA+PVA) HEAT CAPACITY : the specific heat capacity can be defined as the amount of heat required to raise the temperature of 1 g of that substance by 1 degree c . the heat capacity the amount of heat required to raise the temperature of that system by 1 deg c or the rate of change of heat absorbed by the system with increase in temperature . Thermochemistry : the study of heat changes in chemical reactions. Exothermic reaction: the reaction with heat is evolved such as the combustion of aluminum powder. When the reaction is absorbed heat from external heating is called endothermic reaction such as commercial extraction of zinc. Metallurgical processes are usually carried out at constant pressure so that we will consider the heat evolved by the system at constant pressure qp. there are several types of enthalpy such as : heat of reaction, heat of solution, heat of transformation, heat of combustion, heat of formation a compound.