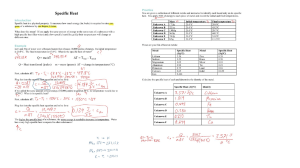
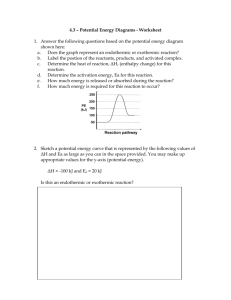
Unit 3: Thermochemistry
advertisement

Belding Area Schools Standard Analysis Unit 3 Chemistry: Thermochemistry STRAND: C SUBJECT: Chem. B Unit 3 STANDARD: See HSCE I can describe conduction in terms of molecules bumping into each other to transfer energy. Explain why there is better conduction in solids and liquids than gases. (C2.2A) I can explain convection and difference in transfer of thermal energy for solids, liquids and gases using evidence that molecules are in constant motion. (C2.2d) I can compare the average kinetic energy of the molecules in a metal object and a wood object at room temperature. (C2.2f) I can calculate the change of H for a given reaction using Hess’s Law. (C3.1a) I can draw enthalpy diagrams for exothermic and endothermic reactions. (C3.1b) I can calculate the change of H for a chemical reaction using simple coffee cup calorimetry. (3.1c) I can calculate the amount of heat produced for a given mass of reactant from a balanced chemical equation. (3.1d) I can describe the energy changes in photosynthesis and in the combustion of sugar in terms of bond breaking and bond making. (C3.2a) I can write chemical equations including the heat term as a part of equation or using the change of H notation. (3.4c) I can draw enthalpy diagrams for reactants and products in endothermic and exothermic reactions. (3.4d) Enduring Understanding(s): Heat is released or absorbed in chemical reactions and is proportional to the amounts of reactants consumed. Some chemical reactions are reversible and involve the same amount of energy regardless of the direction of the reaction. Essential Questions(s): How is energy involved in chemical reactions? Vocabulary: Revised March, 2014 Enthalpy Joule Belding Area Schools Standard Analysis Activation energy Calorie Crystalline solid Delta (change) Endothermic reaction Exothermic reaction Hess’s Law Ionic motion Joule Release of Energy Specific Heat HSCE (High School Content Expectations) See above Information/Rules/Procedures/Resources/Assessments Instructional Strategies for all students: See instructors Moodle page for daily lesson plans, activities, labs, power points, homework, and tutorials. Differentiated Instruction for at-risk students: After school study sessions. Review Guides Tutorials on Moodle Retesting opportunity on all assessments Co-taught classes available Assessments: On staff share drive. Revised March, 2014