9. Names and Formulas for Polyatomic Ions
advertisement

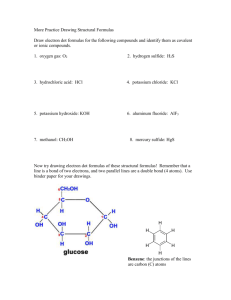
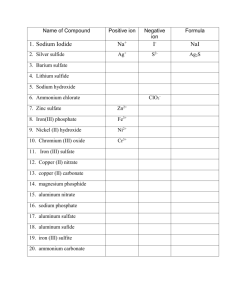
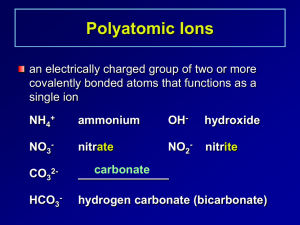
Names and Formulas for Polyatomic Ions 319-1 (II) name and write formulas o some common ionic compounds (both binary and complex), using the periodic table, a list of ions, and appropriate nomenclature for metal and non-metal ions Polyatomic Ions Many ionic compounds are not binary because one or both ions contain atoms of more than one element. These polyatomic ions consist of two or more different non-metal atoms, which are joined by covalent bonds. Copy this chart, it will always be given to you on a test. Name ammonium hydroxide carbonate nitrate sulfate hydrogen carbonate hydrogen sulfate phosphate Chemical Formula NH4+ OHCO3-2 NO3SO4-2 HCO3HSO4PO4-3 Formula to names for polyatomic compounds. NaNO3 Na = sodium NO3= nitrate (look at table) This becomes sodium nitrate Ammonium If you notice on your chart, ammonium is the only cation (it has a positive charge). This means that when you use ammonium, you have to put a non-metal after it. e.g. NH4Cl ammonium chloride More examples (formulas to names) NaHSO4 = sodium hydrogen sulfate CaHCO3 = calcium hydrogen carbonate MgSO4 = magnesium sulfate MgCO3 = magnesium carbonate Try these worksheets on your own! Name to Formula This is where the polyatomic ions get tricky. Pay attention or you will miss something important! Name to Formula When you look at the chart of polyatomic ions, you notice that some have a subscript and a superscript. The superscript tells the charge of the whole group. e.g. NH4+ this has a +1 charge PO4-3 this has a -3 charge HCO3- this has a -1 charge Anytime that you have more than 1 polyatomic ion you use brackets. e.g. Mg(NO3)2 In this example you have 2 nitrate Why? Because Mg has a +2 charge and nitrate has a -1 charge, so you use the xrule and get the subscript Examples of names to formulas sodium nitrate Na+1 NO3 Use the X-rule NaNO3 e.g. magnesium phosphate Mg+2 PO4-3 Because there are more than 1 phosphates, put brackets around PO4 Mg+2 (PO4)-3 Now do the X-rule Mg3(PO4)2 Try the work sheet on your own! P. 119 #6, 7 & 8 ( USE CHART ON PAGE 118)