Polyatomic Ions: Formula Writing & Naming Guide
advertisement

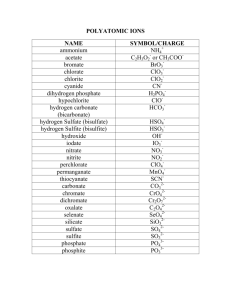
Polyatomic Ions Notes Polyatomic Ions Polyatomic ions are ions that contain two or more atoms. o NH4+ o CO32In a polyatomic ion, the charge belongs to the entire ion. Polyatomic Ions – Formula Writing There is only one difference to writing a chemical formula of a compound containing a polyatomic ion: o If you need more than one polyatomic ion, you put brackets around the polyatomic ion, and place the subscript outside the brackets. Write the formula for Cu+2 and NO3-1 Examples: A. aluminum nitrate B. copper(II) nitrate C. iron (III) hydroxide D. tin(IV) hydroxide Polyatomic Ions – Compound Naming The positive ion is named first. o Check to see if it is a metal with more than one charge. o Check to see if it is polyatomic, for example ammonium (NH4+). The polyatomic ion is then named. o There is an alphabetical list of polyatomic ions on the back of your periodic table. Examples NaNO3: sodium nitrate K2SO4 : potassium sulfate Fe(HCO3)3: iron(III) bicarbonate or iron(III) hydrogen carbonate (NH4)3PO3 ammonium phosphite Use the formula to write the name: 1. MgS 2. MgSO3 3. MgSO4 4. Ca(ClO3)2